Answer:
The
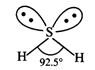
Lewis structure of \[{{H}_{2}}S\] is :
![]() S-atom
is surrounded by four electron pairs (two bonded and two lone pairs). These
four electron pairs adopt tetrahedral arrangement. The presence of two lone
pairs brings distortion in the molecule on account of repulsion with bonded
pairs of electrons. Thus, the shape of \[{{H}_{2}}S\] molecule is
V-shaped
and not linear.
S-atom
is surrounded by four electron pairs (two bonded and two lone pairs). These
four electron pairs adopt tetrahedral arrangement. The presence of two lone
pairs brings distortion in the molecule on account of repulsion with bonded
pairs of electrons. Thus, the shape of \[{{H}_{2}}S\] molecule is
V-shaped
and not linear.
 The
Lewis structure of \[PC{{l}_{3}}\] is :
The
Lewis structure of \[PC{{l}_{3}}\] is :
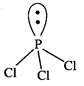
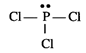 P-atom
is surrounded by four electron pairs (3 bonded and one lone pair). These four
pairs adopt a tetrahedral geometry.
Due
to the presence of lone pair, \[PC{{l}_{3}}\] has a distorted tetrahedral
geometry. Thus, it is pyramidal in shape and not non-planar shape.
P-atom
is surrounded by four electron pairs (3 bonded and one lone pair). These four
pairs adopt a tetrahedral geometry.
Due
to the presence of lone pair, \[PC{{l}_{3}}\] has a distorted tetrahedral
geometry. Thus, it is pyramidal in shape and not non-planar shape.
You need to login to perform this action.
You will be redirected in
3 sec
