Answer:
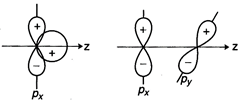
Overlapping
amongst atomic orbitals does not take place if the orientation of atomic
orbitals is not proper i.e., the combining atomic orbitals must have proper
orientation(same symmetry about the molecular axis).
You need to login to perform this action.
You will be redirected in
3 sec
