Answer:
Lewis
Structures
Formal
Charges
\[HN{{O}_{3}}\]
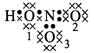
\[H=1-\frac{1}{2}\times
2=0\]
\[{{O}_{1}}=6-(4+\frac{1}{2}\times
4)=0\]\[{{O}_{2}}=6-(4+\frac{1}{2}\times
4)=0\]\[{{O}_{3}}=6-(6+\frac{1}{2}\times 2)=-1\]\[N=5-(\frac{1}{2}\times
8)=+1\]
\[N{{O}_{2}}or{{N}_{2}}{{O}_{4}}\]
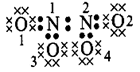
\[{{O}_{1}}\And
{{O}_{2}}=6-(4+\frac{1}{2}\times 4)=0\]\[{{O}_{3}}\And
{{O}_{4}}=6-(6+\frac{1}{2}\times 2)=-1\]\[{{N}_{1}}\And
{{N}_{2}}=5-(\frac{1}{2}\times 8)=+1\]
\[{{H}_{2}}S{{O}_{4}}\]
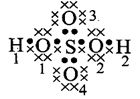
\[{{H}_{1}}\And
{{H}_{2}}=1-\frac{1}{2}\times 2=0\]
\[{{O}_{1}}\And
{{O}_{2}}=6-(4+\frac{1}{2}\times 4)=0\]\[{{O}_{3}}\And
{{O}_{4}}=6-(6+\frac{1}{2}\times 2)=-1\]\[S=6-(\frac{1}{2}\times 8)=+2\]
You need to login to perform this action.
You will be redirected in
3 sec
