Answer:
(a)
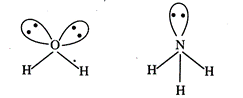 \[{{H}_{2}}O\]has
two lone pairs while \[N{{H}_{3}}\] has single lone pair hence
\[{{H}_{2}}O\]
involves greater lone pair-bond pair repulsion.
\[{{H}_{2}}O\]has
two lone pairs while \[N{{H}_{3}}\] has single lone pair hence
\[{{H}_{2}}O\]
involves greater lone pair-bond pair repulsion.
You need to login to perform this action.
You will be redirected in
3 sec
