Answer:
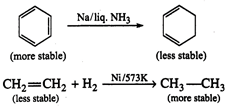
Alkenes and arenes both are
unsaturated and electron rich. Olefins or alkenes undergo addition reaction to
give more stable saturated product; in this reaction hybridization changes from
\[s{{p}^{2}}\]to\[s{{p}^{3}}\].
Arenes are stabilised by
resonance w delocalization of\[\pi \]-electrons. On addition reaction to the
double bond of arene, we get a product which is not resonance stabilised.
Thus, arenes prefer to undergo
substitution reaction while alkenes prefer to undergo addition reaction.
You need to login to perform this action.
You will be redirected in
3 sec
