Answer:
Compound
Planar
ring
Complete
delocalisation of \[\pi \]-electron
Huckel rule \[(4n+2)\pi \]electrons
Aromatic or Non-aromatic
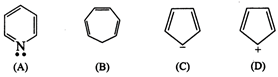
(A)
![]()
Ö
Ö
(\[6\pi e\])Huckel
rule obeyed
Aromatic
(B)
![]()
Ö
x Incomplete
(
![]() Hybrid carbon)
Hybrid carbon)
(\[6\pi e\])
Non-aromatic
(C)
![]()
Ö
Ö
\[6\pi {{e}^{-}}\]
(\[4\pi +2\] lone pair) Huckel rule
verified
Aromatic
(D)
![]()
Ö
x (In-complete)
\[4\pi {{e}^{-}}\]
Non- aromatic
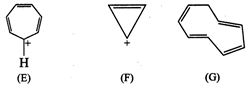
(E)
![]()
Ö
Ö
Ö
Aromatic
(F)
![]()
Ö
Ö
\[2\pi {{e}^{-}}\]Huckel rule verified
for \[n=0\]
Aromatic
(G)
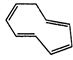
Ö
x
\[8\pi {{e}^{-}}\]Huckel rule not verified
Non- aromatic
You need to login to perform this action.
You will be redirected in
3 sec
