Answer:
(a)\[{{B}_{2}}{{H}_{6}}\]is
an electron deficient compound as it has 12electrons. Each boron atom is linked
with three hydrogen atoms.
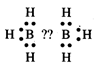 No electron is available to form
a bond between two boron atoms.
No electron is available to form
a bond between two boron atoms.
You need to login to perform this action.
You will be redirected in
3 sec
