Answer:
The hydride is\[NaH\].
\[NaH+{{H}_{2}}O\to
NaOH+{{H}_{2}}\]
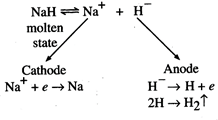 Sodium
and hydrogen are the products of electrolysis of molten\[NaH\].
Sodium
and hydrogen are the products of electrolysis of molten\[NaH\].
You need to login to perform this action.
You will be redirected in
3 sec
