Answer:
(i) \[\overset{\overset{(+2)}{\mathop{+2}}\,}{\mathop{F{{e}^{2+}}}}\,+\overset{\overset{(+12)}{\mathop{+6}}\,}{\mathop{C{{r}_{2}}O_{7}^{2-}}}\,\to
\overset{\overset{(+3)}{\mathop{+3}}\,}{\mathop{F{{e}^{3+}}}}\,+\overset{\overset{(+6)}{\mathop{+3}}\,}{\mathop{C{{r}^{3+}}}}\,\]
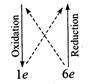 \[6F{{e}^{2+}}+C{{r}_{2}}O_{7}^{2-}\to
6F{{e}^{3+}}+2C{{r}^{3+}}\]
Oxygen and hydrogen will be
balanced by usual methods
\[6F{{e}^{2+}}+C{{r}_{2}}O_{7}^{2-}+14{{H}^{+}}\to
6F{{e}^{3+}}+2C{{r}^{3+}}+7{{H}_{2}}O\](ii) \[\overset{\overset{(0)}{\mathop{0}}\,}{\mathop{{{I}_{2}}}}\,+\overset{\overset{(+5)}{\mathop{+5}}\,}{\mathop{NO_{3}^{-}}}\,\to
\overset{\overset{(+4)}{\mathop{+4}}\,}{\mathop{N{{O}_{2}}}}\,+\overset{\overset{(+10)}{\mathop{+5}}\,}{\mathop{IO_{3}^{-}}}\,\]
\[6F{{e}^{2+}}+C{{r}_{2}}O_{7}^{2-}\to
6F{{e}^{3+}}+2C{{r}^{3+}}\]
Oxygen and hydrogen will be
balanced by usual methods
\[6F{{e}^{2+}}+C{{r}_{2}}O_{7}^{2-}+14{{H}^{+}}\to
6F{{e}^{3+}}+2C{{r}^{3+}}+7{{H}_{2}}O\](ii) \[\overset{\overset{(0)}{\mathop{0}}\,}{\mathop{{{I}_{2}}}}\,+\overset{\overset{(+5)}{\mathop{+5}}\,}{\mathop{NO_{3}^{-}}}\,\to
\overset{\overset{(+4)}{\mathop{+4}}\,}{\mathop{N{{O}_{2}}}}\,+\overset{\overset{(+10)}{\mathop{+5}}\,}{\mathop{IO_{3}^{-}}}\,\]
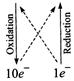 \[{{I}_{2}}+10NO_{3}^{-}\to
10N{{O}_{2}}+2IO_{3}^{-}\]
Oxygen and hydrogen will be
balanced by adding water and \[{{H}^{+}}\] ions.
\[{{I}_{2}}+10NO_{3}^{-}+8{{H}^{+}}\to
10N{{O}_{2}}+2IO_{3}^{-}+4{{H}_{2}}O\]
(iii) \[\overset{\overset{(0)}{\mathop{0}}\,}{\mathop{{{I}_{2}}}}\,+\overset{\overset{(+4)}{\mathop{+2}}\,}{\mathop{{{S}_{2}}O_{3}^{2-}}}\,\to
\overset{\overset{(-2)}{\mathop{-1}}\,}{\mathop{{{I}^{-}}}}\,+\overset{\overset{(+5)}{\mathop{+25}}\,}{\mathop{{{S}_{4}}O_{6}^{2-}}}\,\]
\[{{I}_{2}}+10NO_{3}^{-}\to
10N{{O}_{2}}+2IO_{3}^{-}\]
Oxygen and hydrogen will be
balanced by adding water and \[{{H}^{+}}\] ions.
\[{{I}_{2}}+10NO_{3}^{-}+8{{H}^{+}}\to
10N{{O}_{2}}+2IO_{3}^{-}+4{{H}_{2}}O\]
(iii) \[\overset{\overset{(0)}{\mathop{0}}\,}{\mathop{{{I}_{2}}}}\,+\overset{\overset{(+4)}{\mathop{+2}}\,}{\mathop{{{S}_{2}}O_{3}^{2-}}}\,\to
\overset{\overset{(-2)}{\mathop{-1}}\,}{\mathop{{{I}^{-}}}}\,+\overset{\overset{(+5)}{\mathop{+25}}\,}{\mathop{{{S}_{4}}O_{6}^{2-}}}\,\]
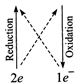 \[{{I}_{2}}+2{{S}_{2}}O_{3}^{2-}\to
2{{I}^{-}}+{{S}_{4}}O_{6}^{2-}\]
(iv)\[\overset{\overset{(+4)}{\mathop{+4}}\,}{\mathop{Mn{{O}_{2}}}}\,+\overset{\overset{(+6)}{\mathop{+3}}\,}{\mathop{{{C}_{2}}O_{4}^{2-}}}\,\to
\overset{\overset{(+2)}{\mathop{+2}}\,}{\mathop{M{{n}^{+}}}}\,+\overset{\overset{(+8)}{\mathop{+4}}\,}{\mathop{C{{O}_{2}}}}\,\]
\[{{I}_{2}}+2{{S}_{2}}O_{3}^{2-}\to
2{{I}^{-}}+{{S}_{4}}O_{6}^{2-}\]
(iv)\[\overset{\overset{(+4)}{\mathop{+4}}\,}{\mathop{Mn{{O}_{2}}}}\,+\overset{\overset{(+6)}{\mathop{+3}}\,}{\mathop{{{C}_{2}}O_{4}^{2-}}}\,\to
\overset{\overset{(+2)}{\mathop{+2}}\,}{\mathop{M{{n}^{+}}}}\,+\overset{\overset{(+8)}{\mathop{+4}}\,}{\mathop{C{{O}_{2}}}}\,\]
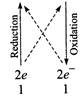 \[Mn{{O}_{2}}+{{C}_{2}}O_{4}^{2-}\to
M{{n}^{2+}}+2C{{O}_{2}}+2{{H}_{2}}O\]
Hydrogen and oxygen can be
balanced as,
\[Mn{{O}_{2}}+{{C}_{2}}O_{4}^{2-}+4{{H}^{+}}\to
M{{n}^{2+}}+2C{{O}_{2}}+2{{H}_{2}}O\]
\[Mn{{O}_{2}}+{{C}_{2}}O_{4}^{2-}\to
M{{n}^{2+}}+2C{{O}_{2}}+2{{H}_{2}}O\]
Hydrogen and oxygen can be
balanced as,
\[Mn{{O}_{2}}+{{C}_{2}}O_{4}^{2-}+4{{H}^{+}}\to
M{{n}^{2+}}+2C{{O}_{2}}+2{{H}_{2}}O\]
You need to login to perform this action.
You will be redirected in
3 sec
