Answer:
(a)
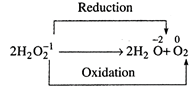 It is an example of
disproportionation in which oxygen is simultaneously oxidised as reduced.
It is an example of
disproportionation in which oxygen is simultaneously oxidised as reduced.
You need to login to perform this action.
You will be redirected in
3 sec
