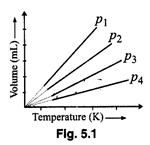
 (a)\[{{p}_{1}}>{{p}_{2}}>{{p}_{3}}>{{p}_{4}}\]
(b) \[{{p}_{1}}={{p}_{2}}={{p}_{3}}={{p}_{4}}\] (c)\[{{p}_{1}}<{{p}_{2}}<{{p}_{3}}<{{p}_{4}}\]
(d) \[{{p}_{1}}<{{p}_{2}}={{p}_{3}}<{{p}_{4}}\]
(a)\[{{p}_{1}}>{{p}_{2}}>{{p}_{3}}>{{p}_{4}}\]
(b) \[{{p}_{1}}={{p}_{2}}={{p}_{3}}={{p}_{4}}\] (c)\[{{p}_{1}}<{{p}_{2}}<{{p}_{3}}<{{p}_{4}}\]
(d) \[{{p}_{1}}<{{p}_{2}}={{p}_{3}}<{{p}_{4}}\]
Answer:
(c) At a fixed
temperature, volume will decrease with increase in pressure (Boyle's law).
Hence, in the given graph
\[{{p}_{1}}<{{p}_{2}}<{{p}_{3}}<{{p}_{4}}\]
You need to login to perform this action.
You will be redirected in
3 sec
