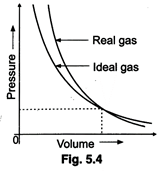
 (i) Interpret the behaviour of
real gas with respect to ideal gas at low pressure.
(ii) Interpret the behaviour of
real gas with respect to ideal gas at high pressure. States of Matter : Gases
and Liquids
(iii) Mark the pressure and
volume by drawing a line at the point where real gas behaves as an ideal gas.
(i) Interpret the behaviour of
real gas with respect to ideal gas at low pressure.
(ii) Interpret the behaviour of
real gas with respect to ideal gas at high pressure. States of Matter : Gases
and Liquids
(iii) Mark the pressure and
volume by drawing a line at the point where real gas behaves as an ideal gas.
Answer:
(i) At low pressure, the curves
of real and ideal gases arevery close, it means at low pressure, the real gases
behave like ideal gas.
(ii) The graph shows that, real
gas is deviating largely from ideal behaviour at high pressure.
(iii) See the dotted lines in
the figure.
You need to login to perform this action.
You will be redirected in
3 sec
