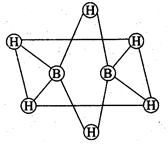
 (a) The two bridged hydrogen
atoms and the two boron atoms lie in one plane.
(b) Out of six B-H bonds two
bonds can be described in terms of 3 centre 2-electron bonds.
(c) Out of six B-H bonds four B-H
bonds can be described in terms of 3 centre 2-electron bonds.
(d) The four terminal B-H bonds
are two centre two electron regular bonds.
(a) The two bridged hydrogen
atoms and the two boron atoms lie in one plane.
(b) Out of six B-H bonds two
bonds can be described in terms of 3 centre 2-electron bonds.
(c) Out of six B-H bonds four B-H
bonds can be described in terms of 3 centre 2-electron bonds.
(d) The four terminal B-H bonds
are two centre two electron regular bonds.
Answer:
(a, b, d) Each of the two
boron atoms is in \[s{{p}^{3}}\]-hybrid state. Of the four hybrid orbitals,
three have one electron each while the fourth is empty. Two of the four
orbitals of each of the boron atom overlap with two terminal hydrogen atoms
forming two normal B?H o-bonds. One of the remaining hybrid orbitals (either
empty or singly occupied) of one of the boron atoms, \[1s\]-orbital of H
(bridge atom) and one of hybrid orbitals of the other boron atom overlap to form
a delocalized orbital covering the three nuclei with pair of electrons. This
is three centre two electron bond.
Similar overlapping occurs with
the second hydrogen atom (bridging) forming three centre two electrons bond.
You need to login to perform this action.
You will be redirected in
3 sec
