Answer:
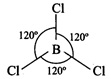
Structure of\[\mathbf{BC}{{\mathbf{l}}_{\mathbf{3}}}\]:
In \[BC{{l}_{3}}\] molecule, the
boron atom is in\[s{{p}^{2}}\] -hybrid state.
Thus, it has planar triangular
structure.
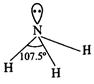
 Structure of\[\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}\]:
In \[N{{H}_{3}}\] molecule, the
nitrogen atom is in \[s{{p}^{3}}\]-hybrid state. Thus, it has tetrahedral
structure having one hybrid orbital with a lone pair of electrons. The actual
shape of the molecule is pyramidal. The bond angle is\[107.5{}^\circ \]. This
is less than the expected angle of\[{{109}^{{}^\circ }}28\]? due to repulsion
between lone pair present on nitrogen atom and bonded pairs of electrons.
Structure of\[\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}\]:
In \[N{{H}_{3}}\] molecule, the
nitrogen atom is in \[s{{p}^{3}}\]-hybrid state. Thus, it has tetrahedral
structure having one hybrid orbital with a lone pair of electrons. The actual
shape of the molecule is pyramidal. The bond angle is\[107.5{}^\circ \]. This
is less than the expected angle of\[{{109}^{{}^\circ }}28\]? due to repulsion
between lone pair present on nitrogen atom and bonded pairs of electrons.
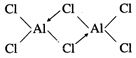
 Structure of \[AlC{{l}_{3}}\]
(dimer):
Al atom undergoes \[s{{p}^{2}}\]
-hybridisation for planar triangular structure. However, to achieve more
stability the vacant p-orbital of Al-atom forms a coordinate bond with one
of the chlorine atom of the other molecule forming a dimer.
Structure of \[AlC{{l}_{3}}\]
(dimer):
Al atom undergoes \[s{{p}^{2}}\]
-hybridisation for planar triangular structure. However, to achieve more
stability the vacant p-orbital of Al-atom forms a coordinate bond with one
of the chlorine atom of the other molecule forming a dimer.
You need to login to perform this action.
You will be redirected in
3 sec
