Answer:
(b) \[w\] (reversible)
< \[w\] (irreversible)
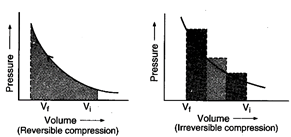 Area under the curve is greater
in irreversible compression than that of reversible compression.
Area under the curve is greater
in irreversible compression than that of reversible compression.
You need to login to perform this action.
You will be redirected in
3 sec
