Answer:
Lattice enthalpy of \[NaCI\]can
be determined by using Born Haber Cycle
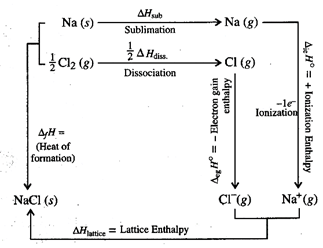 On applying Hess's law:
\[{{\Delta }_{f}}H=\Delta
{{H}_{sub}}+\frac{1}{2}\Delta {{H}_{diss.}}+{{\Delta }_{ie}}H+{{\Delta
}_{eg}}H\] \[+\Delta {{H}_{lattice}}\]
using above relation we can
calculate lattice enthalpy.
On applying Hess's law:
\[{{\Delta }_{f}}H=\Delta
{{H}_{sub}}+\frac{1}{2}\Delta {{H}_{diss.}}+{{\Delta }_{ie}}H+{{\Delta
}_{eg}}H\] \[+\Delta {{H}_{lattice}}\]
using above relation we can
calculate lattice enthalpy.
You need to login to perform this action.
You will be redirected in
3 sec
