Nitrogen Nutrition In Plants
Category : 11th Class
Higher plants generally utilize the oxidized forms such as nitrate \[(NO_{3}^{-})\] and nitrite \[(NO_{2}^{-})\] or the reduced form \[(NH_{4}^{+})\] of nitrogen which is made available by a variety of nitrogen fixers. Nitrogen can be fixed by three methods :
Process of Nitrogen fixation
On the basis of agency through which the nitrogen is fixed the process is divided into two types abiological and biological.
(1) Abiological : They are two types :
(i) Natural or Atmospheric nitrogen fixation : By photochemical and electrochemical reactions, oxygen combines with nitrogen to form oxides of nitrogen. Now they get dissolved in water and combine with other salts to produce nitrates.
Physical nitrogen fixation out of total nitrogen fixed by natural agencies approximately 10% of this occurs due to physical processes such as lightening (i.e., electric discharge), thunder storms and atmospheric pollution.
Due to lightening and thundering of clouds, \[{{N}_{2}}\] and \[{{O}_{2}}\] of the air react to form nitric oxide (NO). The nitric oxide is further oxidised with the help of \[{{O}_{2}}\] to form nitrogen dioxide \[(N{{O}_{2}}).\]
\[{{N}_{2}}+{{O}_{2}}\xrightarrow{\text{Lightening}}2NO\]
\[2NO+{{O}_{2}}\xrightarrow{\text{Oxidation}}2N{{O}_{2}}\]
\[N{{O}_{2}}\]combines with \[{{H}_{2}}O\] to form nitrous acid \[(HN{{O}_{2}})\] and nitric acid \[(HN{{O}_{3}}).\] The acid falls along with rain water. Now it acts with alkaline radicals to form water soluble \[NO_{3}^{-}\] (nitrates) and \[NO_{2}^{-}\](nitrites).
\[2N{{O}_{2}}+{{H}_{2}}O\xrightarrow{{}}HN{{O}_{2}}+HN{{O}_{3}}\]
\[HN{{O}_{3}}+Ca\,\,\,or\,\,K\,\,salts\xrightarrow{{}}Ca\,\,or\,\,K\,\,Nitrates\]
The nitrates are soluble in water and are directly absorbed by the plants.
(ii) Industrial nitrogen fixation : Nitrogen and hydrogen combines to form ammonia industrially, under pressure and temperature.
(2) Biological nitrogen fixation : The conversion of atmospheric nitrogen into inorganic or organic usable forms through the agency of living organisms is called biological nitrogen fixation. The process is carried by two main types of microorganisms, those which are "free living" or asymbiotic and those which live in close symbiotic association of with other plants.
(i) Asymbiotic biological nitrogen fixation : This is done by many aerobic and anaerobic bacteria, cyanobacteria (blue green algae) and some fungi e.g. :
Free living bacteria : Free living N2 fixing bacteria add 10–25 kg of nitrogen /ha/annum.
Aerobic – Azotobacter
Anerobic – Clostridium
Photosynthetic – Chlorobium
Chemosynthetic – Thiobacillis
Cyanobacteria (blue-green algae) e.g., Anabaena, Nostoc, Tolypothrix cylindrospermum, Calotherix and Aulosira etc. They add \[2030kg\] of \[{{N}_{2}}\]per hactare of soil and water bodies.
Free living fungi e.g., Yeast cells and Pullularia.
(ii) Symbiotic biological nitrogen fixation : Symbiotic bacteria are found in the root nodules of the members of family Leguminosae. The best known nitrogen fixing symbiotic bacterium is Rhizobium leguminosarum (Bacillus radicicola).
Rhizobium penetrates to the cortex of root through infection thread. Simultaneously cortical cells or root are stimulated to divide more vigorously to form nodules on the root. Neither bacterium nor plant alone can fix nitrogen in such cases. Nitrogen fixation is actually the outcome of symbiotic relationship between the two. When a section of root nodules is observed the presence of a pigment, leghaemoglobin is seen to impart pinkish colour to it. This pigment is closely related to haemoglobin and helpful in creating optimal condition for nitrogen fixation. Like haemoglobin, leghaemoglobin is an oxygen scavenger. Fixation of nitrogen is done with the help of enzyme nitrogenase, which functions under anaerobic conditions. Leghaemoglobin combines with oxygen and protects nitrogenase.
Symbiotic bacteria (Frankia) have also been found to occur in root nodules of Casuarina, Cycas, Alnus, etc. Leaf nodules develop in some members of family Rubiaceae, the bacteria being Mycobacterium. Some cyanobacteria also have symbiotic association with plants e.g., lichens; Anthoceros (a liverwort) and Azolla (a water fern).
Mechanism of biological nitrogen fixation : Several schemes incorporating such idea have been proposed and Burris (1966) accepts that the total reduction of nitrogen occurs on an enzyme complex (Nitrogenase) without release of intermediates less reduced than ammonia.
The enzyme complex nitrogenase consists of two sub-units
(i) A non-heme iron protein commonly called Fe protein (or dinitrogen reductase, component I).
(ii) An iron molybdenum protein called MoFe protein (or dinitrogenase, component II).
According to Burris (1966) hypothesis for nitrogen fixation suggesting the function of ATP and ferredoxin at each step in the reduction of nitrogen. The pretty function of ATP donor is furnished by pyruvate which also acts as electron donor for \[{{N}_{2}}\]reduction as well.
Pyruvate on one hand acts as ATP donor while on other hand it supplies hydrogen ions and electrons for nitrogen reduction via \[NAD{{H}_{2}}\] and ferredoxin. The nitrogenase enzyme require 16 ATP molecules, 8 hydrogen ions and 8 electrons to reduce one molecule of nitrogen to 2NH3 molecules.
\[{{N}_{2}}+8{{e}^{-}}+8{{H}^{+}}+16ATP\to 2N{{H}_{3}}+H_{2}^{+}+16ADP+16Pi\]
Explaining the mechanism of nitrogenase activity, its now believed that electrons are transferred from the reducing agent (Ferredoxin, Flavoprotein or Dithionite) to complex of Mg-ATP and Fe-protein (component II). From here electrons flow to Mo-Fe protein (component I) and then to substrate (nitrogen) which is finally reduced (to NH3).
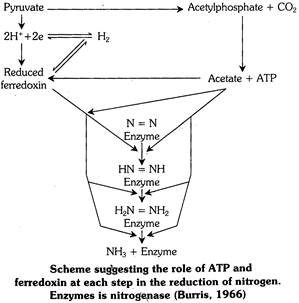
The ammonia formed in biological nitrogen fixation is not liberated. It is highly toxic and is immediately converted into amino acids.
\[Ammonia+\,\,\,\,\alpha -ketoglutarate+\text{ }NADH\xrightarrow{\text{Dehydrogenase}}Glutamate+NA{{D}^{+}}+{{H}_{2}}O.\]
The amino acids are transported through phloem to other parts of the plant.
Ammonification and nitrification
The free living nonsymbiotic nitrogen fixing organisms do not enrich the soil immediately. It is only after organism death that the fixed nitrogen enters the cyclic pool by the two steps namely the ammonification and nitrification.
Ammonification : The nitrogenous organic compounds in the dead bodies of plants and animals are converted into ammonia or ammonium ions in the soil. This is carried out by ammonifying bacteria. Ammonia is toxic to the plants but ammonium ions can be safely absorbed by the higher plants.
Nitrification : Once ammonia has been produced it is converted into nitrates by nitrifying activities and process is called nitrification. Soil bacteria such as Nitrosomonas and Nitrosococcus convert ammonia into nitrite \[(NO_{2}^{-})\] ions.
\[2N{{H}_{3}}+3{{O}_{2}}\xrightarrow{Nitrosomonas\,\,\text{and}\,\,Nitrosococcus}2HN{{O}_{2}}+2{{H}_{2}}O\]
Nitrites are then oxidised to nitrates by Nitrobacter.
\[2HN{{O}_{2}}+{{O}_{2}}\xrightarrow{Nitrobacter}2HN{{O}_{3}}\]
The nitrifying bacteria are chemoautotrophs and are benefited by utilising energy released in oxidation, which is used in chemosynthesis. At soil temperatures \[30{}^\circ C35{}^\circ C\]in alkaline soils and with sufficient moisture and aeration, the activity of ammonifying and nitrifying bacteria is found to be maximum.
Some bacteria such as Thiobacillus denitrificans, Pseudomonas aoruginosa and Micrococcus denitrificans also occur in the soil which convert the nitrate and ammonia into atmospheric free elemental nitrogen. Such bacteria are called denitrifying bacteria and the process is called denitrification. These bacteria act very well in soil where there is more water and less oxygen and there are high level of the carbohydrate.
Nitrate assimilation in plants
It is first reduced to ammonia and then incorporated into organic compounds.
The process of nitrate reduction to ammonia occurs in the following steps :
Nitrate\[\to \]Nitrite\[\to \]Hyponitrite\[\to \]Hydroxylamine\[\to \]Ammonia
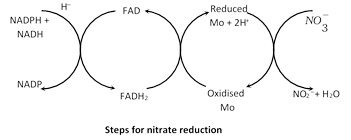
(1) Reduction of nitrate to nitrites : First the nitrate is reduced to nitrite by an enzyme called nitrate reductase. The reductase enzyme is a flavoprotein and contains FAD (Flavin adenine dinucleotide) as prosthetic group which receives hydrogen from reduced NADP or NAD. Molybdenum in enzyme serves as electron carrier.
\[N{{O}_{3}}+NADPH+{{H}^{+}}\underset{FAD/FMN}{\mathop{\xrightarrow{\text{Nitrate}\,\text{reductase}}}}\,NO_{2}^{-}+{{H}_{2}}O+NAD{{P}^{+}}\]
(2) Reduction of nitrites : The nitrite ions are reduced to ammonia by an enzyme called nitrite reductase. This change occurs in leaves in the presence of light more rapidly and in dark with lesser speed. This is due to the reducing power of reaction from photochemical splitting of water.
\[2HN{{O}_{2}}+2{{H}_{2}}O\to 2N{{H}_{3}}+3{{O}_{2}}\]
Nitrite reductase does not need molybdenum but may require the presence of iron and copper. NADH and NADPH act as hydrogen donors.
Application of fertilizers : Most of the soil usually contain sufficient amounts of essential mineral elements for the better crop production. Some of them are, however, deficient in certain elements. These elements are required to be supplemented externally by adding the appropriate fertilizers. Moreover, constant agricultural cultivation in field may also cause depletion of certain elements which must be replenished in order to improve the fertility of soil. The important elements need to be replenished in crop fields are nitrogen, phosphorus and potassium. These are grouped as nitrogenous fertilizers, phosphate fertilizers and potash fertilizers. These are abbreviated as NPK. Common sources of NPK are ammonium chloride, ammonium sulphate, ammonium nitrate, bone meal, calcium magnesium phosphate and nitrate of soda.
You need to login to perform this action.
You will be redirected in
3 sec
