Concept Of Water Relation
Category : 11th Class
Water is mainly absorbed by the roots of the plants from the soil, then it moves upward to different parts and is lost from the aerial parts, especially through the leaves. Before taking up the absorption and movement of water in plants, it is worthwhile to understand the phenomenon of imbibition, diffusion and osmosis involved in the water uptake and its movement in the plants.
Imbibition (L. imbibere – to drink) : The process of adsorption of water by hydrophilic surfaces of a substance without forming a solution is called 'imbibition'. It is a type of diffusion by which movement of water takes place along a diffusion gradient. The solid particles which adsorb water or any other liquid are called imbibants. The liquid which is imbibed is known as imbibate. Agar, cellulose, pectic substances, protoplasmic protein and other organic compound in plant cells show great power of imbibition.
Characteristics of imbibition : The phenomenon of imbibition has three important characteristics :
Volume change : During the process of imbibition, imbibants increase in volume. It has been observed that there is an actual compression of water. This is due to arrangement of water molecules on surface of imbibant and occupy less volume than the same molecules do when are in free stage in the normal liquid. e.g., If a dry piece of wood is placed in water, they swell and increases in its volume.
Production of heat : As the water molecules are adsorbed on the surface of the imbibant, their kinetic energy is released in the form of heat which increases the temperature of the medium. It is called heat of wetting (or heat of hydration). e. g., during kneading, the flour of wheat gives a warm feeling due to imbibition of water and consequent release of heat.
Development of imbibitional pressure : Imbibition pressure can be defined as the maximum pressure that an imbibant will develop when it is completely soaked in pure water. Imbibition pressure is also called as the matric potential because it exists due to the presence of hydrophilic substances in the cell which include organic colloids and cell wall.
Factors influencing the rate of imbibition
Nature of imbibant : Proteins are the strongest imbibants of water, starch less strong, cellulose being the weakest.
Surface area of imbibant : If more surface area of the imbibant is exposed and is in contact with liquid, the imbibition will be more.
Temperature : Increase in temperature causes an increase in the rate of imbibition.
Degree of dryness of imbibant : If the imbibant is dry it will imbibe more water than a relatively wet imbibant.
Concentration of solutes : Increase in the concentration of solutes in the medium decreases imbibition.
pH of imbibant : Proteins, being amphoteric in nature, imbibe least in neutral medium. Towards highly acidic or highly alkaline pH, the imbibition increases till a maximum is reached, there after it starts slowing down.
Significance of imbibition
(1) The water is first imbibed by walls of root hairs and then absorbed.
(2) Water is absorbed by germinating seeds through the process of imbibition and helps in rupturing of seed coat (made up of cellulose).
(3) The water moves into ovules which are ripening into seeds by the process of imbibition.
Diffusion : The movement of the molecules of gases, liquids and solids from the region of higher concentration to the region of lower concentration is known as diffusion.
It may occur between gas and gas (e.g., diffusion of ammonia into air), liquid and liquid (e.g., diffusion of alcohol into water), or solid and liquid (e.g., diffusion of sugar into water).
Diffusion pressure : It is a hypothetical term coined by Meyer (1938) to denote the potential ability of the molecules or ions of any substance to diffuse from an area of their higher concentration to that of their lower concentration.
Diffusion pressure deficit (DPD) or Suction pressure (SP) : The term diffusion pressure (DP) and diffusion pressure deficit (DPD) were putforth by B.S. Meyer in 1938. Now a days, the term water potential (y) is used which is equal to DPD, but negative in value. The term suction pressure putforth by Renner (1915).
The amount by which the diffusion pressure of water or solvent in a solution is lower than that of pure water or solvent is known as diffusion pressure deficit (DPD)'. Diffusion pressure deficit is the water absorbing capacity of a solution. Therefore, DPD can also be called suction pressure (SP).
Factors influencing rate of diffusion
Temperature : Increase in temperature leads to increase in the rate of diffusion.
Pressure : The rate of diffusion of gases is directly proportional to the pressure. So the rate of diffusion increases with increase in pressure. Rate of diffusion µ pressure.
Size and mass of diffusing substance : Diffusion of solid is inversely proportional to the size and mass of molecules and ions.
Rate of diffusion \[\propto \frac{1}{\text{Size}\times \text{Mass}\,\text{of}\,\text{particles}}\]
Density of diffusing substance : The rate of diffusion is inversely proportional to the square root of density of the diffusing substance. Larger the molecules, slower will be the rate of diffusion. This is also called Graham's law of diffusion.
\[D\propto \frac{1}{\sqrt{d}}\](D = Diffusion and d = Density of diffusing substance).
According to the density the diffusion of substances takes place in following manner :
Gas > Liquid > Solid
The vapours of volatile liquids (scent or petrol) and solids (camphor) also diffuse like gases.
Density of the medium : The rate of diffusion is slower, if the medium is concentrated. Thus, a gas would diffuse more rapidly in vacuum than in air. Substances in solution also diffuse but at a much slower rate than gases.
Diffusion pressure gradient (DPG) : The rate of diffusion is directly proportional to the difference of diffusion pressure at the two ends of a system and inversely proportional to the distance between the two.
Significance of diffusion
(1) Gaseous exchange during the processes of photosynthesis and respiration takes place with the help of diffusion.
(2) The process of diffusion is involved in the transpiration of water vapours.
(3) Aroma of flowers is due to diffusion of volatile aromatic compounds to attract pollinating animals.
(4) During passive salt uptake, the ions are absorbed by process of diffusion.
(5) Diffusion helps in translocation of food materials.
(6) Gaseous exchange in submerged hydrophytes takes place by general surface of the cells through diffusion.
Permeability : Permeability is the degree of diffusion of gases, liquids and dissolved substances through a membrane. Different types of membranes may be differentially permeable to different substances.
Types of membranes : On the basis of permeability.
Permeable membrane : These membranes allow free passage of solvent (water) and most of the dissolved substances. e.g., cell wall in plant cells. Filter paper is made up of pure cellulose it also functions as permeable membrane.
Impermeable membrane : This type of membranes with deposits of waxy substances like cutin and suberin, do not allow the entry of water, dissolved substances and gases. e.g., suberized walls of cork cells, cuticle layer of leaf.
Semi-permeable membrane : These membranes permit the movement of solvent molecules only through them, but prevent the movement of solute particles. e.g., egg membrane, animal bladder, parchment paper, copper ferrocyanide membrane, membranes of collodion.
Selectively or Differentially permeable membrane : This type of membranes allow selective passage of solutes along with solvent, through them.
Many biological membranes such as cell membrane (plasmalemma), tonoplast (vacuolar membrane) and the membranes surrounding the sub-cellular organelles are selectively permeable. A non-living selectively permeable membrane is cellophane.
Osmosis : Osmosis (Gr. Osmos = a pushing or impulse) was discovered by Abbe Nollet in 1748 and also coined the term 'osmosis'. First of all Traube (1867) used copper ferrocyanide and developed semipermeable membrane to show its utility in the osmosis of plant physiology. First time Pfeffer in (1887) developed osmoscope by using semipermeable membrane.
Osmosis is special type of diffusion of a liquid, when solvent moves through a semipermeable membrane from a place of higher diffusion pressure to a place of lower diffusion pressure.
or
It is the migration of solvent from a hypotonic solution (of lower concentration) to hypertonic solution (of higher concentration) through a semi-permeable membrane to keep the concentration equal.
In for malin preserved spirogyra filament, selective permeability of plasma membrane is lost and hence no effect on placing in hypertonic solution.
Reverse Osmosis : It is the reverse movement of water through a semipermeable membrane from a more concentrated solution to a more dilute solution by applying external pressure on the more concentrated solution.
It is used in removing salts from saline water as well as extra – purification of water.
Osmotic pressure (OP) : Pfeffer coined the term osmotic pressure.
Osmotic pressure is that equivalent of maximum hydrostatic pressure which is produced in the solution, when this solution is separated from its pure solvent by a semipermeable membrane.
Types of osmosis : Depending upon the movement of water into or outside of the cell, osmosis is of two types.
Endosmosis : The osmotic flow of water into a cell, when it is placed in a solution, whose solute concentration is less than that of the cell sap, is called endosmosis e.g., swelling of raisins, when they are placed in water.
When a fish of marine water kept in fresh water then it will die due to endosmosis.
An animal cell placed in pure water will swell up and bursts.
Pollen grains of some of plants germinate on stigma soon but they burst in water or dilute sugar solution.
Exosmosis : The osmotic outflow of water from a cell, when it is placed in a solution, whose solute concentration is more than that of the cell sap, is called exosmosis. e.g., shrinkage of grapes, when they are placed in strong sugar solution.
Osmotic concentrations (Types of solutions)
Hypotonic solution (hypo = less than). A solution, whose osmotic concentration (solute potential) is less than that of another solution or cell sap is called hypotonic solution. If a cell is placed in such a solution, water starts moving into the cell by the process of endosmosis, and cell becomes turgid.
Hypertonic solution (hyper = more than). A solution, whose osmotic concentration (solute potential) is more than that of another solution or cell sap is called hypertonic solution. If a cell is placed in such a solution, water comes out of the cell by the process of exosmosis and cell becomes flaccid. If potato tuber is placed in concentrated salt solution it would becomes shrink due to loss of water from its cell.
Isotonic solution (iso = the same). A solution, whose osmotic concentration (solute potential) is equal to that of another solution or cell sap, is called isotonic solution. If a cell is placed in isotonic solution, there is no net changes of water between the cell and the solution and the shape of cell remain unchanged.
In xerophytes, the osmotic concentration of cell sap is more than the normal. The osmotic pressure of given solution can be calculated by following formula.
Osmotic pressure = CST
Where, C = Molar concentration of solution, S = Solution constant, which is 0.082 and T = Absolute temperature i.e., \[273{}^\circ K.\]
Significance of osmosis in plants
(1) The phenomenon of osmosis is important in the absorption of water by plants.
(2) Cell to cell movement of water occurs throughout the plant body due to osmosis.
(3) The rigidity of plant organs (i.e., shape and form of organism) is maintained through osmosis.
(4) Leaves become turgid and expand due to their OP.
(5) Growing points of root remain turgid because of osmosis and are thus, able to penetrate the soil particles.
(6) Opening and closing of stomata is affected by osmosis.
(7) Movement of plants and plant parts, e.g., movement of leaflet of Indian telegraph plant.
Turgor pressure (TP) : The plant cell, when placed in pure water, swells but does not burst. Because of negative osmotic potential of the vacuolar solution (cell sap), water will move into the cell and will cause the plasmalemma be pressed against the cell wall. The actual pressure that develops that is the pressure responsible for pushing the membrane against cell wall is termed turgor pressure.
Significance of turgidity in plants
(1) It provides stability to a cell.
(2) Turgidity keeps the cell and their organelles (mitochondria, plastids and microbodies) fully distended. This is essential for plants to live and grow normally.
(3) Turgor pressure helps in cell enlargement, consequently in stretching of the stems and in keeping leaves erect and fully expanded.
(4) The turgid cells provide mechanical support necessary for the non woody tissues (maize, sugarcane, banana etc.).
(5) Loss of turgidity leads to wilting of leaves and drooping of shoots.
(6) The opening and closing of stomata are regulated by the turgidity of the guard cells.
(7) Leaf movements (seismonastic movement) of many plants (such as bean, sensitive plant Mimosa pudica) are controlled by loss and gain of cell turgor.
(8) Due to turgor pressure plumule and radicles force out from seeds at the time of seed germination.
Wall pressure (WP) : Wall pressure (WP) may, therefore, be defined as 'the pressure exerted by the cell wall over the protoplast to counter the turgor pressure. Normally wall pressure is equal and opposite to turgor pressure (WP =TP) except when the cell become flaccid. The value of the two forces continue to rise with the continued entry of water, till the cell becomes fully turgid.
Interrelationship of DPD (S.P.), OP and TP (WP) : DPD indicates the sucking power of suction pressure. As water enters into the cell the TP of the cell is increased. Cell wall exerts equal and opposite WP against TP. The actual force responsible for entry of water will be therefore OP–TP
i.e., \[DPD=OPWP\text{ (}As\text{ }WP=TP\text{)}\]
\[DPD=OPTP\]
Consider that a plant cell with \[OP=10\]atm. is immersed in pure water. In the beginning TP inside the cell is zero i.e.,
\[DPD=OP=10\text{ }atm.\]
When water enters into the cell, TP increases. Turgidity increases and cell wall develops equal and opposite WP. At the stage of equilibrium \[TP=10\] atm. and DPD will become zero. It is important to note that OP was same when cell was flaccid and turgid.
\[DPD=OPTP\]
\[=100=10\](when flaccid)
\[=1010=0\](when turgid)
The entry of water in cell to cell depends upon the DPD and not on OP and TP. This can be examplified as follows :
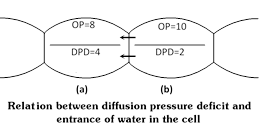
Since the DPD of cell A is more, it has less water and, therefore water would diffuse from cell B into the cell A (because that DPD of cell B is less than that of A or it has more water than cell A has). The entry of water into the cell A would stop when DPD of both the cells become equal. In this way water moves from a cell with less DPD into the cell with more DPD. Thus, DPD is the osmotic parameter, which determines the flow of water from one cell to another.
Under given suitable conditions, the DPD is more than OP when TP is negative.
Plasmolysis (Gr. Plasma = something formed; lysis = loosing) : "The shrinkage of the protoplast of a living cell from its cell wall due to exosmosis under the influence of a hypertonic solution is called plasmolysis". The stage of plasmolysis, when the protoplast just begins to contract away from the cell wall is called incipient plasmolysis. The stage when the cell wall has reached its limit of contraction and the protoplast has detached from cell wall attaining spherical shape is called evident plasmolysis. If a cell with incipient plasmolysis is placed in a hypertonic solution it will show more plasmolysis.
Deplasmolysis : "The swelling up of a plasmolysed protoplast due to endosmosis under the influence of a hypotonic solution or water is called deplasmolysis'. Deplasmolysis is possible only immediately after plasmolysis otherwise the cell protoplast becomes permanently damaged. The value of TP becomes zero at the time of limiting plasmolysis and below zero during incipient and evident plasmolysis. Leaf of Tradescantia is used for demonstration of plasmolysis in laboratory.
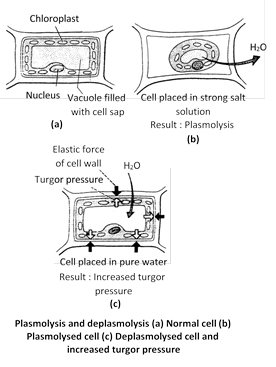
Significance of plasmolysis
(1) The OP of a cell can be measured by plasmolysis. The OP of a cell is roughly equal to the OP of a solution that causes incipient plasmolysis in the cell.
(2) Salting of pickles, meat, fishes etc. and addition of sugar to jams, jellies, cut fruits etc., prevent their decay by microbes, as the latter get killed due to plasmolysis or due to high concentration of salt or sugar.
(3) By salting, the weeds can be killed from tennis courts and the growth of plants can be prevented in the cracks of walls.
(4) Plasmolysis is helpful in determining whether a particular cell is living or dead as plasmolysis does not occur in a dead or non living cell.
Water potential (y) : The term water potential was coined by slatyer and Taylor (1960). It is a modern term which is used in placed of D.P.D. The movement of water in plants cannot be accurately explained in terms of difference in concentration or in any other linear expression. The best way to express spontaneous movement of water from one region to another is in terms of the difference of free energy of water between two regions. Free energy is the thermodynamic parameter, that determine the direction in which physical and chemical changes must occur. The potential energy of water is called water potential. e.g., water is stored behind a dam. When the water runs downhill, its potential energy can be converted to electrical energy. This conversion of energy of water is due to gravity. The other source that provides energy to water is pressure. The increasing pressure increases the free energy there by increasing water potential.
Water running downhill due to gravity can be made to run uphill by overcoming the water potential (energy) by applying pressure. This means that water moves from the point, where water potential is greater to the other, where water potential is less. The difference in water potential between two points is a measure of the amount of work or energy needed to move water from one point to the other. Thus, based on the concept of water potential, the direction of water movement can be predicted. Water potential is measured in terms of pressure.
Measurement unit of water potential is pascal, Pa (1 mega pascal, Mpa = 10 bars). It is represented by Greek letter, Psi (y). Water potential (yw) is the difference between chemical potential of water at any point in a system (mw) and that of pure water under standard conditions (mw°). The value of water potential can be calculated by formula :
\[{{\psi }_{w}}+(\mu m)-(\mu m)=RT\,\,1\,\,n\,\,e/e{}^\circ \]
where \[{{\psi }_{w}}=\] water potential, R is gas constant, T is absolute temperature (K), e is the vapour pressure of the solution in the system at temperature T, and e° the vapour pressure of pure water at the same temperature.
The direction in which water will move from one cell to another cell depends on water potential in two regions. Water potential is measured in bars. A bar is a pressure unit which equals \[14.5\text{ }lb/i{{n}^{2}},\,750\,mm\text{ }Hg\]or 0.987 atm.
Water potential of pure water at normal temperature and pressure is zero. This value is considered to be the highest. The presence of solute particles reduces the free energy of water and thus decreases the water potential. Therefore, water potential of a solution is always less than zero or has negative value.
Component of water potential : The water potential \[(\psi )\] in a plant cell or tissue can be written as the sum of the matric potential \[(\psi \,m)\] due to binding of water to cell walls and cytoplasm, the solute potential \[(\psi \,s)\] due to concentration of dissolved solutes, which by its effect on the entropy components reduces the water potential and the pressure potential \[(\psi \,p)\] due to hydrostatic pressure, which by its effect on the energy components increases the water potential :
\[\psi =\psi m+\psi s+\psi p\] …… (1)
Matric potential \[\mathbf{(\psi m)}\] : Matric is the term used for the surface (such as, soil particles, cell walls, protoplasms, etc.) to which water molecules are adsorbed. The matric potential (y m) is the component of water potential influenced by the presence of a matrix. It has got a negative value. In case of plant cells and tissues, the matric potential is often disregarded because it is not significant in osmosis. Thus, the above equation (1) may be simplified as follows :
\[\psi =\psi s+\psi p\] …… (2)
In normal cells of mesophytes and hydrophytes it is almost negligible.
Solute potential \[\mathbf{(\psi s)}\] : Solute potential is also known as Osmotic potential. It is defined as the amount by which the water potential is reduced as a result of the presence of solute. Solute potentials or osmotic potentials \[(\psi s)\] are always in negative values (number). The term solute potential takes the place of osmotic pressure \[(\pi ;\,\,pi)\] expressed in bars with a negative sign.
\[{{\psi }_{s}}=-\pi \]
Pressure potential \[\mathbf{(\psi p)}\] : Plant cell wall is elastic and it exerts a pressure on the cellular contents. As a result of inward wall pressure, hydrostatic pressure is developed in the vacuole termed as turgor pressure. The pressure potential is usually positive and operates in plant cells as wall pressure and turgor pressure.
Its magnitude varies between +5 bars (during day) and +15 bars (during night).
Physical states of cell : Three physical states of cell, according to their water potential, are as follows :
In case of fully turgid cell : In case of fully turgid cell, the net movement of water into the cell is stopped. The cell is in equilibrium with the water outside. The water potential in such a case will be zero (0).
Water potential = Osmotic potential + Pressure potential
\[\psi =\psi s+\psi p\]
A cell at full turgor has its osmotic potential and pressure potential equal but opposite in sign. Therefore, its water potential will be zero. For example, supposing a cell has its \[\psi s\] of \[10\] bars and \[\psi p\]of 10 bars the resultant water potential will be zero as follows :
\[\psi =\psi s+\psi p\]
\[\psi =10\text{ }bars+10\text{ }bars\]
\[\psi =0\text{ }bars\]
In case of flaccid cell : When a plant cell is flaccid, its turgor becomes zero (corresponding to a turgor pressure of a 0 bars). Zero turgor is approached under natural conditions when a tissue is severely wilted. A cell at zero turgor has an osmotic potential \[(\psi s)\] equal to its water potential \[(\psi ).\] For example, supposing a flaccid cell has an osmotic potential of \[10\] bars and pressure potential \[(\psi p)\] of 0 bars.
Water potential = Osmotic potential + Pressure potential
\[\psi =\psi s+\psi p\]
\[\psi =10\text{ }bars+0\text{ }bars\]
\[\psi =10\text{ }bars\]
The water potential of the cell will be \[10\text{ }bars,\] which is less as compared to the water potential of pure water (0 bars).
In case of plasmolysed cell : When the vacuolated parenchymatous cells are placed in solutions of sufficient strength the protoplast decreases in volume to such an extent that they shrink away from the cell wall. The cells are plasmolysed. Such cells have negative value of pressure potential (negative turgor pressure). The resultant water potential will be more negative, as for example, a plasmolysed cell has osmotic potential of \[10\] bars and pressure potential of \[2\] bars the water potential of the cell will be \[12\]bars.
Water potential = Osmotic potential + Pressure potential
\[\psi =\psi s+\psi p\]
\[\psi =-10+(-2)\]
\[\psi =-12\,\,bars\]
Movement of water between two adjacent cells : Suppose A and B are two adjacent plant cells where osmotic movement of water can occur. Cell A has osmotic potential \[(\psi s)\] of \[-16\] bars and pressure potential of 8 bars. The cell B has osmotic potential of \[-12\] bars and pressure potential of 2 bars. The movement of water will be as follows :
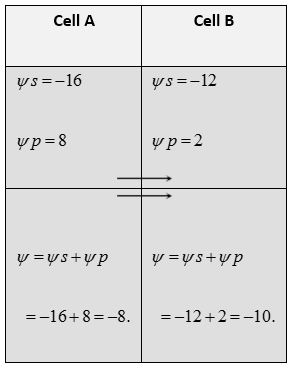
Wilting : A plant usually fails to survive if it is conditioned to water deficiency. The symptoms appear in the plant, plant parts or in the cells due to scarcity of water are termed as wilting. It is loss of turgidity causing folding and drooping of leaves and other soft aerial parts of the plant. It is of three types :
(1) Incipient wilting : There is no external symptoms but the mesophyll cells lose a part of their water content during midday due to transpiration.
(2) Temporary wilting : It occurs during midday and is visible externally due to drooping of leaves and young shoots. At noon the rate of transpiration is quite high as compared to water absorption, which decreases further due to depletion of water around rootlets. It is corrected in the afternoon when transpiration decreases.
(3) Permanent wilting : It is the last stage in wilting when the aerial parts do not regain turgidity even if placed in water saturated atmosphere. It is caused by decrease in water content of the soil which increases TSMS (Total soil moisture stress) or resistance to absorption to such an extent that plant roots are unable to absorb water. Permanent Wilting Percentage (PWP) is the percentage of water on the dry weight basis of the soil that is present in the soil when the plants growing in it first touch the condition of permanent wilting. This value varies between \[115%\] and depends upon the texture of the soil e.g., clay has higher PWP than sand.
You need to login to perform this action.
You will be redirected in
3 sec
