Types of respiration
Category : 11th Class
On the basis of the availability of oxygen and the complete or incomplete oxidation of respiratory substrate. The respiration may be either of the following two types : Aerobic respiration and Anaerobic respiration
Aerobic respiration
It uses oxygen and completely oxidises the organic food mainly carbohydrate (Sugars) to carbon dioxide and water. It therefore, releases the entire energy available in glucose.
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}+6{{O}_{2}}\xrightarrow{enzymes}6C{{O}_{2}}+6{{H}_{2}}O+energy\,\,(686Kcal)\]
It is divided into two phases : Glycolysis, Aerobic oxidation of pyruvic acid.
Glycolysis / EMP pathway
(1) Discovery : It was given by Embden, Meyerhof and Parnas in 1930. It is the first stage of breakdown of glucose in the cell.
(2) Definition : Glycolysis ( Gr. glykys= sweet, sugar; lysis= breaking) is a stepped process by which one molecule of glucose (6c) breaks into two molecules of pyruvic acid (3c).
(3) Site of occurrence : Glycolysis takes place in the cytoplasm and does not use oxygen. Thus, it is an anaerobic pathway. In fact, it occurs in both aerobic and anaerobic respiration.
(4) Inter conversions of sugars : Different forms of carbohydrate before entering in glycolysis get converted into simplest form like glucose, glucose 6-phosphate or fructose 6-phosphate. Then these sugars are metabolized into the glycolysis.
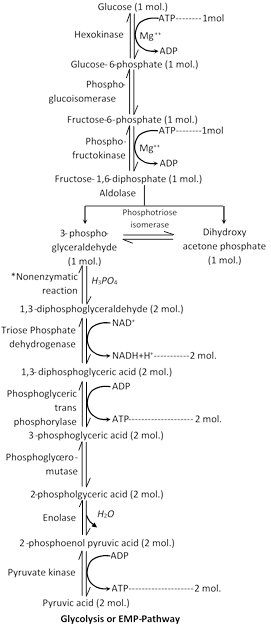
(5) Special features of glycolysis : The special features of glycolysis can be summarised as follows :
(i) Each molecule of glucose produces 2 molecules of pyruvic acid at the end of the glycolysis.
(ii) The net gain of ATP in this process is two ATP molecules (four ATPs are formed in glycolysis but two of them are used up in the reaction).
(iii) During the conversion of 1, 3-diphosphoglyceraldehyde into 1, 3-diphosphoglyceric acid one molecule of \[NAD{{H}_{2}}\]is formed. As each molecule of glucose yields two molecules of 1,3-diphosphoglyceric acid, hence each molecule of glucose forms 2 molecules of \[NAD{{H}_{2}}.\]
(iv) During aerobic respiration (when oxygen is available) each \[NAD{{H}_{2}}\] forms 3 ATP and \[{{H}_{2}}O\] through electron transport system of mitochondria. In this process \[{\scriptscriptstyle 1\!/\!{ }_2}\,\,{{O}_{2}}\] molecule is utilized for the synthesis of each water molecule.
In this way during aerobic respiration there is additional gain of 6 ATP in glycolysis
\[\underset{(\text{net}\,\text{gain})}{\mathop{2ATP}}\,+\underset{\text{(addition}\,\text{gain)}}{\mathop{6ATP}}\,\to \underset{\text{(total}\,\text{net}\,\text{gain)}}{\mathop{8ATP}}\,\]
(v) Reaction of glycolysis do not require oxygen and there is no output of \[C{{O}_{2}}.\]
(vi) Formation of 1, 3- diphosphoglyceraldehyde called non enzymatic phosphorylation.
(vii) Overall reaction of glycolysis represented by following reaction :
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\to \underset{\text{Pyruvate}}{\mathop{2{{C}_{3}}{{H}_{4}}{{O}_{3}}}}\,+4H\]
Total input and output materials in glycolysis
|
Total Input |
Total Output |
|
1 molecule of glucose (6 C) |
2 molecules of pyruvate \[(2\times 3\,\,C)\] |
|
\[2\text{ }ATP\] |
\[4\text{ }ATP\] |
|
\[4\text{ }ADP\] |
\[2\text{ }ADP\] |
|
\[2\times NA{{D}^{+}}\] |
\[2\times NADH+2{{H}^{+}}\] |
|
\[2\text{ }Pi\] |
\[2\times {{H}_{2}}O\] |
Aerobic oxidation of pyruvic acid
(1) Oxidative decarboxylation of pyruvic acid : If sufficient \[{{O}_{2}}\] is available, each 3-carbon pyruvate molecule \[(C{{H}_{3}}COCOOH)\] enters the mitochondrial matrix where its oxidation is completed by aerobic means. It is called gateway step or link reaction between glycolysis and Kreb's cycle.
Decarboxylation and dehydration :
\[\underset{\text{(Pyruvic acid)}}{\mathop{C{{H}_{3}}CO.COOH}}\,+\underset{\text{(CoA)}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CoA.SH+NAD}}\,\underset{\begin{smallmatrix} **TPP \\ **LAA \end{smallmatrix}}{\mathop{\xrightarrow{\begin{smallmatrix} \text{Pyruvic}\,\text{dehydrogenase} \\\text{multienzyme}\,\text{complex} \end{smallmatrix}}}}\,\underset{\text{(acetyl-S-CoA)}}{\mathop{C{{H}_{3}}.CO.S.CoA}}\,+NAD.2H+C{{O}_{2}}\underset{\text{(acetyl-S-CoA)}}{\mathop{C{{H}_{3}}.CO.S.CoA}}\,+NAD.2H+C{{O}_{2}}\]**TPP=Thiamine pyrophosphate**LAA=Lipoic acid amide
Acetyl CoA is a common intermediate of carbohydrate and fat metabolism. Latter this acetyl CoA from both the sources enters Kreb's cycle. This reaction is not a part of Kreb's cycle.
(2) Kreb's cycle / TCA cycle / Citric acid cycle
Discovery : This cycle has been named after the German biochemist in England Sir Hans Krebs who discovered it in 1937. He won Noble Prize for this work in 1953. Krebs cycle is also called the citric acid cycle after one of the participating compounds. It takes place in the mitochondrial matrix.

Summary of Kreb's cycle
(i) All the enzymes, reactants, intermediates and products of TCA cycle also are found in aqueous solution in the matrix, except the succinate dehydrogenase (mitochondrial marker enzyme) which is located in the inner mitochondrial membrane.
(ii) Oxidation of one mole of acetyl CoA uses 4 molecules of water and releases one molecule of water.
(iii) Liberates 2 molecules of carbon dioxide.
(iv) Gives off 4 pairs of hydrogen atoms.
(v) Produces one GTP/ ATP molecule during the formation of succinate.
(vi) One mole of acetyl CoA gives 12 ATP during oxidation in Krebs cycle.
(vii) Regenerates oxaloacetate used in last cycle for reuse.
The above summary is for one molecule of acetyl coenzyme A. There are two acetyl coenzyme A molecules formed from one molecule of glucose by glycolysis and oxidative decarboxylation of pyruvate.
Difference between Glycolysis and Kreb's cycle
| Glycolysis | Kreb's cycle |
|
It takes place in the cytoplasm. |
It takes place in the matrix of mitochondria. |
|
It occurs in aerobic as well as anaerobic respiration. |
It occurs in aerobic respiration only. |
|
It consists of 9 steps. |
It consists of 8 steps. |
|
It is a linear pathway. |
It is a cyclic pathway. |
|
It oxidizes glucose partly, producing pyruvate. |
It oxidises acetyl coenzyme A fully. |
|
It consumes 2 ATP molecules. |
It does not consume ATP |
|
It generates 2 ATP molecules net from 1 glucose molecules. |
It generates 2 GTP/ATP molecules from 2 succinyl coenzyme A molecules. |
|
It yields 2 NADH per glucose molecule. |
It yields 6 NADH molecules and 2 FADH2 molecules from 2 acetyl coenzyme A molecules. |
|
It does not produce \[C{{O}_{2}}.\] |
It produces \[C{{O}_{2}}.\] |
|
All enzyme catalysing glycolytic reactions are dissolved in cytosol. |
Two enzymes of Krebs cycle reactions are located in the inner mitochondrial membrane, all others are dissolved in matrix. |
Product formed during aerobic respiration by Glycolysis and Kreb's cycle.
Total formation of ATP
| ATP formation in Glycolysis | |||
|
|
Steps |
Product of reactions |
In terms of ATP |
|
ATP formation by substrate phosphorylation |
1, 3-diphosphoglyceric acid (2 moles)\[\to \] 3 phosphoglyceric acid (2 moles) Phosphoenolpyruvic acid (2 moles)\[\to \] Pyruvic acid (2 moles) |
2 ATP 2 ATP |
2 ATP 2 ATP |
|
|
|
Total |
4 ATP |
|
ATP formation by oxidative phosphorylation or ETC |
1, 3 - diphosphoglyceraldehyde (2 moles) 1, 3 - diphosphoglyceric acid (2 moles) |
\[2\text{ }NAD{{H}_{2}}\] |
6 ATP |
|
|
Total ATP formed |
\[4+6\text{ }ATP\text{ }=\] |
10 ATP |
|
ATP consumed in Glycolysis |
Glucose (1 mole)\[\to \]Glucose 6 phosphate (1 mole) Fructose 6 phosphate (1 mole)\[\to \] Fructose 1, 6-diphosphate (1 mole) |
\[\text{ }1\text{ }ATP\] \[\text{ }1\text{ }ATP\] |
\[\text{ }1\text{ }ATP\]
\[\text{ }1\text{ }ATP\] |
|
|
|
Total |
2 ATP |
|
|
Net gain of ATP = total ATP formed ? Total ATP consumed |
10 ATP ? 2ATP |
8 ATP |
|
ATP formation in Kreb's cycle |
|||
|
ATP formation by substrate phosphorylation |
Succinyl CoA (2 mols)\[\to \] Succinic acid (2 mols) |
2 GTP |
2 ATP |
|
|
|
Total |
2 ATP |
|
ATP formation by oxidative phosphorylation or ETC |
Pyruvic acid (2 mols)\[\to \] Acetyl CoA (2 mols) Isocitric acid (2 mols)\[\to \] Oxalosuccinic acid (2 mols) a-Ketoglutaric acid (2 mols)\[\to \] Succinyl CoA (2 mols) Succinic acid (2 mols)\[\to \] Fumaric acid (2 mols) Malic acid (2 mols)\[\to \] Oxaloacetic acid (2 mols) |
\[2\text{ }NAD{{H}_{2}}\]
\[2\text{ }NAD{{H}_{2}}\]
\[2\text{ }NAD{{H}_{2}}\]
\[2\text{ }NAD{{H}_{2}}\]
\[2\text{ }NAD{{H}_{2}}\] |
6 ATP *
6 ATP
6 ATP
4 ATP
6 ATP |
|
|
|
Total |
28 ATP |
|
|
Net gain in Kreb's cycle (substrate phosphorylation + oxidative phosphorylation) |
2ATP + 28 ATP |
30 ATP |
|
Net gain of ATP in glycolysis and Kreb's cycle |
Net gain of ATP in glycolysis + Net gain of ATP in Kreb's cycle |
8 ATP + 30 ATP |
38 ATP |
|
Over all ATP production by oxidative phosphorylation or ETC |
ATP formed by oxidative phosphorylation in glycolysis + ATP formed by oxidative phosphorylation or ETC. |
6 ATP + 28 ATP |
34 ATP |
22 ATP produced by oxidation of \[NAD{{H}_{2}}\] and \[FAD{{H}_{2}}\] in Kreb’s cycle and 6 ATP comes from oxidative decarboxylation of pyruvic acid.
These ATPs are not included neither in glycolysis nor kreb's cycle.
Formation and use of water
|
Formation of water molecules |
||
|
Formation of water molecules in glycolysis |
2 phosphoglyceric acid (2 mols) \[\xrightarrow{-{{H}_{2}}O}\]2 phosphoenol pyruvic acid (2 mols) 1, 3-diphosphoglyceraldehyde \[\xrightarrow{-{{H}_{2}}O}\]1, 3 diphosphoglyceric acid |
\[2{{H}_{2}}O\]
\[2{{H}_{2}}O\] |
|
|
Total water molecules formed in glycolysis |
\[4{{H}_{2}}O\] |
|
Formation of water molecules in kreb?s cycle |
One molecule of water in each of the five oxidation reactions (these reactions occur twice as there are two molecules of pyruvic acid). Other than oxidation reaction Citric acid (2 mols) ® Cis-aconitic acid (2 mols) |
\[10\,{{H}_{2}}O\]
\[2\,{{H}_{2}}O\] |
|
|
Total water molecules formed in Kreb?s cycle |
\[12\,{{H}_{2}}O\] |
|
|
Total water molecules formed in aerobic respiration (Glycolysis + Kreb's cycle including activation of pyruvates) |
\[16\,{{H}_{2}}O\] |
|
Use of water molecules |
||
|
Use of water in Glycolysis |
3-phosphoglyceraldehyde (2 mols) \[\xrightarrow{+{{H}_{2}}O}\] 1, 3 diphosphoglyceric acid (2 mols) |
\[2\,{{H}_{2}}O\] |
|
|
Total water molecule used in glycolysis |
\[2\,{{H}_{2}}O\] |
|
Use of water in Kreb?s cycle |
Oxaloacetic acid (2 mols) \[\xrightarrow{+{{H}_{2}}O}\] Citric acid (2 mols) Cis aconitic acid (2 mols) \[\xrightarrow{+{{H}_{2}}O}\] Isocitric acid (2 mols) Succinyl CoA (2 mols) \[\xrightarrow{+{{H}_{2}}O}\] Succinic acid (2 mols) Fumaric acid (2 mols) \[\xrightarrow{+{{H}_{2}}O}\] Malic acid (2 mols) |
\[2\,{{H}_{2}}O\]\[2\,{{H}_{2}}O\] \[2\,{{H}_{2}}O\] \[2\,{{H}_{2}}O\] |
|
|
Total water molecules used is Kreb's cycle |
\[8\,{{H}_{2}}O\] |
|
|
Total water molecules used in aerobic respiration (Glycolysis + Kreb?s cycle) |
\[10\,{{H}_{2}}O\] |
|
Net gain of water molecules in aerobic respiration |
Number of water molecules formed - Number of water molecules used\[=(16{{H}_{2}}O10{{H}_{2}}O)\] |
\[6\,{{H}_{2}}O\] |
Evolution of carbon dioxide
|
Pyruvic acid (2 mols) \[\xrightarrow{-C{{O}_{2}}}\] Acetyl CoA (2 mols) Oxalosuccinic acid \[\xrightarrow{-C{{O}_{2}}}\,\,\alpha \] ketoglutaric acid (2 mols) a Ketoglutaric acid (2 mols) \[\xrightarrow{-C{{O}_{2}}}\] Succinyl CoA (2 mols) |
\[2\,C{{O}_{\mathbf{2}}}\] \[2\,C{{O}_{\mathbf{2}}}\] \[2\,C{{O}_{\mathbf{2}}}\] |
|
Total \[C{{O}_{\mathbf{2}}}\]molecules released in aerobic respiration |
\[6\,C{{O}_{\mathbf{2}}}\] |
Use of \[{{O}_{2}}\](Oxygen)
|
Use of oxygen in Glycolysis |
1, 3-diphosphoglyceraldehyde (2mols) \[\xrightarrow{+\frac{1}{2}{{O}_{2}}}\] 1, 3-diphosphoglyceric acid (2 mols) |
\[1\,{{O}_{2}}\] |
|
Use of oxygen in Kreb's cycle |
Five oxidation reactions of Kreb's cycle (2 times) |
\[5\,{{O}_{2}}\] |
|
|
Total \[{{O}_{2}}\] molecules required for aerobic respiration |
\[6\,{{O}_{2}}\] |
Energy storage and energy transfer : In respiration energy released takes in the form of chemical energy, stored in a form called ATP. Energy transfer of biological oxidation hinges on the formation of labile high energy phosphate bonds of ATP. Nicotinamide adenine dinucleotide phosphate (NAD), Flavin adenine dinucleotide (FAD), Guanosine triphosphate are also the product of respiration and converted to ATP by electron transport system.
Adenosine triphosphate
There are several compounds like NAD, FAD, GTP and ATP are known as energy yielding compounds. The best known, and probably the most important of these are adenosine triphosphate (ATP). It serves as the energy currency of the cells.
Structure of ATP : Adenosine triphosphate is a nucleotide consisting of three main constituents;
(i) A nitrogen contain purine base (Adenine).
(ii) A five carbon sugar ribose.
(ii) Three inorganic phosphate groups.
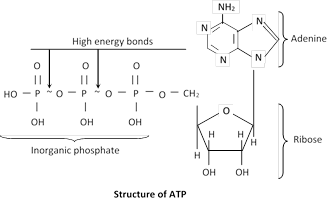
The bonds attaching the last two phosphate to the rest of the molecule are high energy bonds (~) contain more than twice the energy of an average chemical bond.
ATP hydrolysis : The energy is usually released from ATP by hydrolysing the terminal phosphate groups.
\[\text{Adenosine}\,\text{triphosphate }\xrightarrow{\text{hydrolysis}}\text{Adenosine}\,\text{diphosphate}\,(ADP)\underset{{}}{\mathop{+Pi+}}\,7.3Kcal.....\]
\[\text{Adenosine}\,\text{diphosphate}\xrightarrow{\text{hydrolysis}}\text{Adenosine}\,\text{monophosphate(AMP)}+Pi+7.3Kcal.\]
Phosphorylation : The ATP hydrolysis reactions are reversible because ATP are synthesized from ADP, Pi and energy (take up for the bond formation).
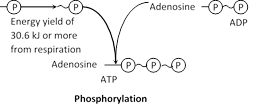
The addition of phosphate group to ADP and AMP called phosphorylation. Energy required for the bond formation is equal to the energy released in hydrolysis. The significant role of ATP as an intermediate energy transfer compound.
Major functions of ATP : ATP molecules receive the energy, which released in exergonic reactions and make this energy available for various endergonic reactions. Some of the important process in which ATP is utilized are as follows:
(i) Synthesis of carbohydrates, proteins, fats, etc.
(ii) Translocation of organic food.
(iii) Absorption of organic and inorganic food.
(iv) Protoplasmic streaming.
(v) Growth.
Nicotinamide adenine dinucleotide phosphate/ Nicotinamide adenine dinucleotide (NADP/NAD) : It is called universal hydrogen acceptor, produced during aerobic respiration (glycolysis+ Kreb's cycle) and also in anaerobic respiration, work as coenzyme in ATP generation Via electron transport system. NADP have one additional phosphate.
NAD plays a crucial role in dehydrogenation processes. Some dehydrogenases do not work with NAD, but react with NADP (Nicotinamide adenine dinucleotide phosphate). Formerly called Coenzyme II or Triphosphopyridine nucleotide = TPN Nicotinamide is a vitamin of B group.
First NAD and NADP both functions as hydrogen acceptors. Later H ions and electrons \[({{e}^{\_}})\] from these are transported through a chain of carriers and after being released at the end of a chain react with \[{{O}_{2}}\] and from \[{{H}_{2}}O\] (see Electron Transport chain). During the release of 2 electron from \[2{{H}^{+}}\] atoms from NAD. 2H and their reaction with \[{{O}_{2}}\] to form water, 3 ATP molecules are synthesized.
(3) Electron transport system : The electron transmitter system is also called electron transport chain (ETC), or cytochrome system (CS), as five out of these nine carriers are cytochrome. It is the major source of cells energy, in the respiratory breakdown of simple carbohydrates intermediates like phosphoglyceraldehyde, pyruvic acid, isocitric acid, \[\alpha -\]ketoglutaric acid, succinic acid and malic acid are oxidised. The oxidation in all these brought about by the removal of a pair of hydrogen atoms (2H) from each of them. This final stage of respiration is carried out in ETS, located in the inner membrane of mitochondria (in prokaryotes the ETS is located in mesosomes of plasma membrane). The system consists of series of precisely arranged nine electron carriers (coenzyme) in the inner membrane of the mitochondrion, including the folds or cristae of this membrane. These nine electron-carriers function in a specific sequence and are :
Nicotinamide adenine dinucleotide (NAD), Flavin mononucleotide (FMN), Flavin adenine dinucleotide (FAD), Co-enzyme-Q or ubiquinone, Cytochrome-b, Cytochrome\[{{c}_{1}},\] Cytochrome-c, Cytochrome-a and Cytochrome-a3,
The first carrier in the chain is a flavoprotein which is reduced by \[NAD{{H}_{2}}.\] Coenzyme passes these electron to the cytochromes arranged in the sequence of \[b-{{c}_{1}}-c-a-{{a}_{3}},\]finally pass the electron to molecular oxygen. In this transport, the electrons tend to flow from electro-negative to electro-positive system, so there is a decrease in free energy and some energy is released so amount of energy with the electrons goes on decreasing. During electron-transfer, the electron-donor gets oxidised, while electron-acceptor gets reduced so these transfers involve redox-reaction and are catalysed by enzymes, called reductases. Oxidation and reduction are complimentary. This oxidation-reductiion reaction over the ETC is called biological oxidation.
\[\text{Electron}-\text{donor}\xrightarrow{{{\text{e}}^{-}}}\,\text{electron}-\text{acceptor}\]
here, electron-donor and electron –acceptor form redox pair.
During the electron transfers, the energy released at some steps is so high that ATP is formed by the phosphorylation of ADP in the presence of enzyme ATP synthetase present in the head of \[{{F}_{1}}-\]particles present on the mitochondrial crista. This process of ATP synthesis during oxidation of coenzyme is called oxidative phosphorylation, so ETS is also called oxidative phosphorylation pathways.
\[\text{ADP}+\text{Pi}\xrightarrow{\text{ATP Synthetase}}\text{ATP}\]
From the cytochrome a3, two electrons are received by oxygen atom which also receives two proton \[({{H}^{+}})\] from the mitochondrial matrix to form water molecule. So the final acceptor electrons is oxygen. So the reaction
\[{{H}_{2}}+\frac{1}{2}{{O}_{2}}\to {{H}_{2}}O\] (called metabolic water) is made to occur in many steps through ETC, so the most of the energy can be derived into a storage and usable form.
(i) Two route systems of ETC : The pairs of hydrogen atoms from respiratory intermediates are received either by \[NA{{D}^{+}}\]or FAD coenzymes which becomes reduced to \[NAD{{H}_{2}}\] and \[FAD{{H}_{2}}.\]These reduced coenzyme pass the electrons on to ETC. Thus, regeneration of NAD+ or FAD takes place in ETC. There are two routes ETC :
(a) Route 1 :\[NAD{{H}_{2}}\] passes their electrons to Co-Q through FAD . In route 1 FAD is the first electron carrier. 3 ATP molecules are produced during the transfer of electron on following steps :
NAD to FAD
Cyt b to \[Cyt\text{ }{{c}_{1}}\]and
Cyt a to \[Cyt\text{ }{{c}_{3}}\]
(b) Route 2 : \[FAD{{H}_{2}}\]passes their electron directly to FAD. 2 ATP molecules are produced during the transfer of electron on following steps.
Cyt b to \[Cyt\text{ }{{c}_{1}}\]and
Cyt a to \[Cyt\text{ }{{c}_{3}}\]
(ii) Structure of mitochondria in relation to oxidative function : On inner side of mitochondria elementary particles or\[{{F}_{0}}-{{F}_{1}}\]complex of ATPase complex or elementary particle (oxysomes) are found. Previously it was considered that elementary particles contain all the enzyme of oxidative phosphorylation and electron transport chain.
Component of electron transport chain are located in the inner membrane in the form of respiratory chain complexes. For complexes following theories are given :
(a) Four complex theory : According to Devid green electron transport chain contains 4 complexes-
Complex I : Comprises NADH dehydrogenase and its 6 Iron Sulphur centers (Fe-S).
Complex II : Consists of Succinate dehydrogenase and its 3 Iron Sulphur centers.
Complex III : Consists of cytochrome b and c, and a specific Iron-Sulphur centers.
Complex IV : Comprises cytochromes a and a3.
(b) Five complex theory : According to Hatefi, (1976), Complex I to Complex IV are related to the electron transport.
Complex II : Succinate : CoQ reductase
Complex III : Reduced \[CoQ\text{ }\,\text{(}CoQ{{H}_{2}}\text{)}\]: cytochrome C reductase
Complex IV : Cytochrome C oxidase
Complex V : ATPase
Cytochrome C and Q are mobile components of the respiratory chain.
(iii) Oxidative phosphorylation : The process of ATP synthesis during oxidation of reduced coenzymes in ETC is called oxidative phosphorylation.
Peter Mitchell (1961) proposed the chemiosmotic mechanism of ATP synthesis (Noble prize in 1978) which states that ATP synthesis occurs due to \[{{H}^{+}}-\]flow through a membrane. It involves two steps :
(a) Development of proton gradient. At each step of ETC, the electron- acceptor has a higher electron –affinity than the electron-donor. The energy from electron-transport is used to move the proton \[({{H}^{+}})\] from the mitochondrial matrix to inter-membranous or outer chamber. Three pairs of protons are pushed to outer chamber during the movement of electrons along route I while two pairs of protons are moved to outer chamber during the movement of electrons along route–II. This generates a pH-gradient across the inner mitochondrial membrane with protons \[({{H}^{+}})\] concentration higher in the outer chamber than in the mitochondrial matrix. This difference in H+ concentration across the inner mitochondrial membrane is called proton-gradient(\[\Delta \]pH). Due to proton gradient, an electrical potential \[(\Delta \psi )\] is developed across the inner mitochondrial membrane as the matrix is now electronegative with respect to the intermembranous (outer) chamber. The proton gradient and membrane electric potential collectively called proton motive force.
(b) Proton flow : Due to proton-gradient, the protons returns to the matrix while passing through proton channel of \[{{F}_{0}}-{{F}_{1}}\] ATPase. This proton gradient activates the enzyme ATP synthetase or \[{{F}_{0}}{{F}_{1}}\] ATPase.
ATP synthetase controls the formation of ATP from ADP and inorganic phosphate in the presence of energy.
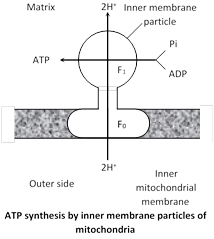
(iv) Role of shuttle system in energy production : Glycolysis occurs in the cytoplasm outside the mitochondrion in which \[2NAD{{H}_{2}}\] molecules are produced but ETC is located along inner mitochondrial membrane, so \[NAD{{H}_{2}}\] of glycolysis must enter inside the mitochondrion to release energy. But the inner mitochondrial membrane is impermeable to \[NAD{{H}_{2}}.\] In mitochondrial membrane, there are 2 shuttle-system, each formed of carrier-molecule.
These shuttle systems are :
(a) Malate-Aspartate shuttle : When this electron shuttle occurs, transfer of electrons from \[NADP{{H}_{2}}\]in cytoplasm occurs to NAD inside the mitochondria. This is more efficient and result in production of 38 ATP molecules.
(b) Glycerol-Phosphate shuttle : In this shuttle transfer of electrons from \[NAD{{H}_{2}}\] in cytoplasm occurs to FAD inside mitochondria and it results in production of 36 ATP molecules. It is less efficient and results in the reduction of FAD inside the mitochondrion.
Which shuttle predominates depends on the particular species and tissues envolved, for example : 38 ATP are formed in kidney, heart and liver cell while 36 ATP molecules are formed in muscle cells and nerve cells. In these cells glycerol-phosphate shuttle is predominant and 2 ATP formed from \[NAD{{H}_{2.}}\].
You need to login to perform this action.
You will be redirected in
3 sec
