Biogeochemical Cycle
Category : 12th Class
Organisms are built up on chemical substances. They require certain chemicals like \[{{N}_{2}},{{O}_{2}},{{H}_{2}},P,C,\] etc. continuously for their survival. These chemicals enter the organisms from the environment and come out after undergoing changes or without changes. Thus these elements tend to circulate in a characteristic path from the environment to the organism and back to the environment. This cyclical path of the elements from the abiotic system to the biotic system and back is called biogeochemical cycles (Bio = living organism; Geo = water, air, earth). As these chemicals form the components of food, these cycles are also called nutrient cycles.
Phases of biogeochemical cycles : Each biogeochemical cycle has two phases, namely the biotic phase (organic phase) and the abiotic phase.
(1) Biotic phase : It refers to the flow of chemicals in the living organisms through food chain.
(2) Abiotic phase : It refers to the distribution and flow of chemicals in the non-living environment.
Types of biogeochemical cycles : The biogeochemical cycles are classified into two types, namely gaseous cycles and sedimentary cycles.
(1) Gaseous cycles : In gaseous cycles the main reservoirs of chemicals are the atmosphere and ocean. e.g., Carbon cycle, \[{{N}_{2}}\] cycle, \[{{O}_{2}}\] cycle, etc.
(2) Sedimentary cycle : In sedimentary cycles the main reservoirs are soil and rocks. e.g., Sulphur cycle, phosphorus cycle, etc.
Important biogeochemical cycles
(1) Carbon Cycle : The cycling of carbon between biotic and abiotic systems is called carbon cycle. It is a gaseous cycle. The main source of carbon is the carbon dioxide \[(C{{O}_{2}}).\,\,C{{O}_{2}}\] is present in the air and water. Air is the main reservoir. \[C{{O}_{2}}\] content of air is 0.03%. Its amount remains constant.

(i) Flow of Carbon into the biotic system : Carbon flows into the biotic system in two ways :
(a) Photosynthesis : Carbon enters the biotic system through photosynthesis. In photosynthesis green plants utilize \[C{{O}_{2}}\] and incorporate the carbon of \[C{{O}_{2}}\] in glucose. Glucose is used for the synthesis of other types of carbohydrates, proteins and lipids. These compounds, containing carbon, are stored up in the plant tissues. When plants are eaten up by herbivores, the carbon flows into the body of herbivorous animals through food chain. When herbivores are eaten by carnivores, the carbon enters the body of carnivorus animals.
\[6C{{O}_{2}}+6{{H}_{2}}O\to {{C}_{6}}{{H}_{12}}{{O}_{6}}+\text{ }6{{O}_{2}}.\]
(b) Formation of shell : The \[C{{O}_{2}}\] dissolved in sea water is utillized by the marine animals like protozoans, corals, molluscs, algae, etc., for the construction of shell. In these animals \[C{{O}_{2}}\] is converted into calcium carbonate \[(CaC{{O}_{3}})\] which is used for the construction of shells.
\[C{{O}_{2}}+{{H}_{2}}O\to {{H}_{2}}C{{O}_{3}}\](Carbonic acid)
\[{{H}_{2}}C{{O}_{3}}\to {{H}^{+}}+HC{{O}_{3}}\](Bicarbonate)
\[HC{{O}_{3}}+C{{a}^{+}}\to {{H}^{+}}+CaC{{O}_{3}}\](Calcium carbonate)
(ii) Flow of Carbon into the abiotic system : The carbon of the biotic system flows into the abiotic system in five ways:
(a) Respiration : Plants and animals release \[C{{O}_{2}}\] by respiration (biological oxidation).
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\to C{{O}_{2}}+{{H}_{2}}O+\]Energy
(b) Decomposition : When plants and animals die, the dead bodies are decomposed into \[C{{O}_{2}}\] by decomposers like bacteria, algae, etc.
(c) Shells : After the death of marine animals, \[CaC{{O}_{3}}\] stored in the shells is either deposited as sedimentary rocks or dissolved in water to release \[C{{O}_{2}}\] by the reversion of the above said reactions.
(d) Coal : A certain proportion of carbon from plants is deposited as coal. Carbon from coal returns to air in the form of \[C{{O}_{2}}\] through combustion and weathering.
(e) Forest fire : Combustion of wood in the forest, releases carbon from plants in the form of \[C{{O}_{2}}.\]
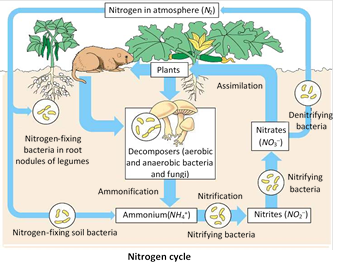
(2) Nitrogen cycle : The cycling of nitrogen between abiotic and biotic systems is called nitrogen cycle. It is a gaseous cycle. The main source of N2 is air which contains 79% \[{{N}_{2}}.\]
(1) Flow of Nitrogen into the biotic system : Nitrogen is an important nutrient of plants. But plants cannot utilize free \[{{N}_{2}}\]of air. They obtain N2 from ammonium salts, nitrites and nitrates. These compounds are formed from atmospheric \[{{N}_{2}}\] by a process called nitrogen fixation.
Nitrogen fixation is a process by which atmospheric free \[{{N}_{2}}\] is converted into soluble salts like nitrites and nitrates. It occurs in two ways namely electrochemical fixation and biological fixation.
(a) Electrochemical fixation : A certain amount of free \[{{N}_{2}}\] is fixed by the action of lightning. The amount of nitrate formed by this method is about \[35\,\,mg/{{m}^{2}}/year.\]
(b) Biological fixation : It refers to the conversion of free \[{{N}_{2}}\] into soluble salts by the activity of certain organisms. These organisms are called \[{{N}_{2}}\] fixing organisms. The amount of nitrate formed by this method is about 140 to \[700\text{ }mg/{{m}^{2}}/\,\]year, and in a fertile area it exceeds \[20000\,\,mg/m.\] The \[{{N}_{2}}\] fixing organisms are bacteria, blue green algae, fungi and other micro-organisms. e.g., Rhizobium, Azotobacter, Closteridium, Bacillus, Nitrosomonas, Nitrococcus, Nitrobacter, Anabena, Nostoc, etc.
The fixed \[{{N}_{2}}\] is absorbed by plants through the root system and is incorporated into the proteins. When herbivores feed on these plants, the \[{{N}_{2}}\] flows on the carnivores through food chain.
(2) Flow of Nitrogen into the abiotic system : The nitrogen of the biotic system flows into the abiotic system by four methods, namely decomposition, excretion, denitirfication and sedimentation.
(a) Decomposition : Plants and animals contain nitrogen in their body protein. After death, the proteins of dead bodies are decomposed by decomposers into amino acids and ammonia. The convertion of protein from dead bodies into ammonia by decomposition is called ammonification. This ammonia may be converted into nitrates or free nitrogen.
![]()
(b) Excretion : Animals excrete nitrogenous waste products in the form of ammonia, urea and uric acid. These compounds are decomposed to release \[{{N}_{2}}.\]
(c) Denitrification : The conversion of nitrate into ammonia or free nitrogen is called denitrification. This is done by denitrifying bacteria. e.g., Pseudomonas.These bacteria utilize the \[{{O}_{2}}\] present in the nitrate for the oxidation of carbohydrate.
(d) Sedimentation : Some amount of nitrate is lost from the ecosystem by sedimentation.
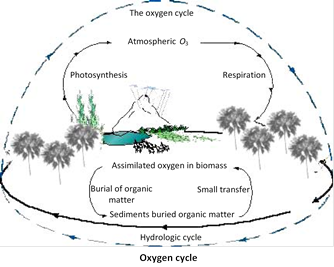
(3) Oxygen cycle : The cycling of \[{{O}_{2}}\] between biotic and abiotic systems is called \[{{O}_{2}}\] cycle. It is a gaseous cycle. Air is the reservoir for \[{{O}_{2}}.\,\,{{O}_{2}}\] enters the biosphere through respiration. The \[{{O}_{2}}\] taken into the body is used for oxidation of carbohydrates, proteins and fats. Certain amount of \[{{O}_{2}}\] in atmospheric air is converted into ozone \[({{O}_{3}})\] the ozone forms an umbrella-like layer in the outer atmosphere. This layer prevents the ultraviolet radiations from reaching the earth's surface.
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\to 6CO+6{{H}_{2}}O+\]Energy
\[{{O}_{2}}+O\to {{O}_{3}}\]
Carbon monoxide is released from volcanoes. This CO is unstable. It combines with \[{{O}_{2}}\] to form \[C{{O}_{2}}.\]
\[{{O}_{2}}\]combines with a variety of elements to form compounds. For example, it forms \[C{{O}_{2}}\] with carbon, water with hydrogen, nitrates with \[{{N}_{2}}\] ferric oxide with iron etc. \[{{O}_{2}}\] returns to air by two main methods, namely photosynthesis and photodissociation.
\[{{O}_{2}}\ +\ C\ \to \ C{{O}_{2}}\]
\[{{O}_{2}}+\ 2{{H}_{2}}\ \to \ 2{{H}_{2}}O\]
\[{{O}_{2}}\ +\ {{N}_{2}}\ \to \ N{{O}_{3}}\]
(i) Photosynthesis : Green plants synthesize carbohydrate by photosynthesis. During photosynthesis water molecules break up into hydrogen and oxygen. \[{{O}_{2}}\] is released into the atmosphere and H2 is trapped and turned into carbohydrates.
\[12{{H}_{2}}O+6C{{O}_{2}}\to {{C}_{6}}{{H}_{12}}{{O}_{6}}+6{{H}_{2}}O+6{{O}_{2}}\]
(ii) Photodissociation : Water vapour is dissociated to release \[{{H}_{2}}\] and \[{{O}_{2}},\] in presence of light.
(4) Phosphorus cycle : The cycling of phosphorus between biotic and abiotic system is called phosphorus cycle. It is a sedimentary cycle. Phosphorus is an important mineral nutrient. The main source of phosphorus is rocks. Through erosion and weathering phosphorus is made available in the soil. Plants absorb ionic phosphate through roots. In plants it is incorporated into the protoplasmic components like DNA, RNA, AMP, ADP, ATP, GDP, GTP, NADP, phospholipids etc. from plants, it passes into herbivores and animals, the organic molecules containing phosphate are decomposed and phosphate is liberated as inorganic ion phosphate. It is again used by plants.
The excess of phosphate in the bodies of animals is excreted out through faces. The bird guano (excreta) contains a large amount of phosphate. Phosphate is also released to the soil through the combustion of forest trees and grasses. A large amount of phosphate is lost in the sea by sedimentation. A certain amount of phosphorus gets locked in bones and teeth.

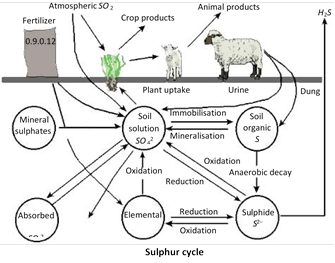
(5) Sulphur cycle : The cycling of sulphur between biotic and abiotic systems is called sulphur cycle. It is a sedimentary cycle. Sulphur is an important component of proteins and amino acids.
Sulphur exists in a number of states. Of these, three are important. They are elemental sulphur, sulphides and sulphates. Sulphur is present in rocks. It is made available for plants in the form of inorganic sulphate by weathering and erosion. Sulphur passes into the animals through food chain. By the death of plants and animals, the decomposers again bring the sulphur to the soil for the use of plants.
Some sulphur in dead bodies is released into the air as hydrogen sulphide \[({{H}_{2}}S)\] by the bacteria called Escherichia coli under anaerobic combustion. Similarly incomplete combustion of fossil fuel releases sulphur dioxide \[(S{{O}_{2}})\] into the air.
Certain bacteria (green and purple photosynthetic bacteria) oxidise \[{{H}_{2}}S\]of air to sulphate which can be used by plants.
\[{{H}_{2}}S+2{{O}_{2}}\to S{{O}^{}}_{4}+\text{ }2{{H}^{+}}\]
Certain amount of sulphur is lost in the sediments. If iron is present in the sediments, sulphur combines with it to form iron sulphide.
\[Fe+S\to FeS\]
You need to login to perform this action.
You will be redirected in
3 sec
