Chemical Bonding
Category : 7th Class
Atoms of the elements lose and gain electrons to form new substances. The process, by which they form the new substances, is called chemical bonding. An atom of a substance is made up of three fundamental particles, i.e. electrons, protons and neutrons. Out of three particles, protons and electrons are charged whereas neutron does not possess any charge. Protons are positively charged and electrons are negatively charged. Two like charges repel each other and two unlike charges attract each other. Negatively charged electrons orbiting the nucleus, whereas protons are present in the nucleus.
The atoms, which lose electrons in a chemical bonding is called positive ions or cations. The atoms, which gain electrons are called negative ions or anions. For example, reaction between sodium (Na) and chlorine ![]() leads to the transfer of electrons from Na to
leads to the transfer of electrons from Na to ![]() to form
to form ![]() cations and
cations and ![]() anions. The bonding of atoms of the chemicals mainly depends on their charges. The force between the two charged particles is called electromagnetic force. The force between atoms allows the formation of chemical compound. The strength of the chemical bonding of the two atoms is the actual strength of electromagnetic force between them. Look at the Following Picture of Formation of Salt from the Reaction of Sodium and Chlorine
anions. The bonding of atoms of the chemicals mainly depends on their charges. The force between the two charged particles is called electromagnetic force. The force between atoms allows the formation of chemical compound. The strength of the chemical bonding of the two atoms is the actual strength of electromagnetic force between them. Look at the Following Picture of Formation of Salt from the Reaction of Sodium and Chlorine



Sodium metal Chlorine Gas Formation of slat Table salt
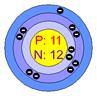
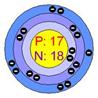
Atomic structure of sodium Atomic structure of chloride
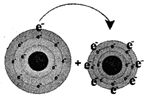

Sodium loses one electron Sodium chloride (table salt)
In the picture (1), sodium loses its one valance electron and gives it to chlorine. In the picture (2) the atoms of the resulting elements are positively charged sodium ions and negatively charged chlorine ions. The bond, between sodium and chlorine is called ionic bond. Because, in this reaction, one reactant is in metallic form and another is in nonmetallic form. The atomic number of sodium is 11 and the valance electron in outer most orbits is 1. The atomic number of chlorine is 17 and the number of valance electrons in outer most orbits is 7.
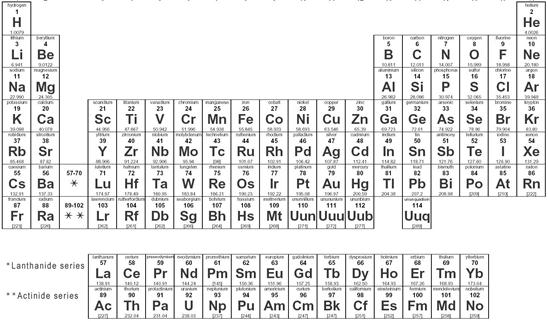
For the classification of atomic structure of known elements a table has been introduced, which is called periodic table.
Periodic Table
In the periodic table the elements have been arranged according to their atomic number. The atomic number of an element is the number of protons in its nucleus.
The columns of periodic table signify the group of the elements. The elements are grouped by shearing its common property. In a group, all elements have equal valance electrons. The roman numeral on the top of the table shows the number of valance electrons in the group.
Look at the Following Picture of Periodic Table

![]() Which one of the following particles are exchanged from one atom to another during a chemical reaction?
Which one of the following particles are exchanged from one atom to another during a chemical reaction?
(a) Neutrons
(b) Protons
(c) Electrons
(d) All of these
(e) None of these
Answer: (c)
![]() The atomic number of a substance is its number of protons in nucleus. Which one of the following is the atomic number of potassium?
The atomic number of a substance is its number of protons in nucleus. Which one of the following is the atomic number of potassium?
(a) 17
(b) 18
(c) 19
(d) 20
(e) None of these
Answer: (c)
You need to login to perform this action.
You will be redirected in
3 sec
