Monohydric Alcohols
Category : JEE Main & Advanced
These are compound containing one hydroxyl group. Their general formula is \[{{C}_{n}}{{H}_{2n+2}}O\]
(1) Preparation : (i) From alkyl halide
\[\underset{\text{Bromoethane}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,\,+\underset{\text{(Aqueous)}}{\mathop{KOH}}\,\to \underset{\text{Ethanol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,+KBr\]
\[\underset{\text{Bromoethane}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,\,\,\,+\underset{\text{Moist silver oxide}}{\mathop{AgOH}}\,\to \,\,\,\,\underset{\text{Ethanol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,+AgBr\]
\[\underset{\,\,Br}{\overset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,+\underset{\text{(Aqueous)}}{\mathop{KOH}}\,\to \underset{\begin{smallmatrix}\text{2-Methylpropene} \\\text{ (Major product)}\end{smallmatrix}}{\mathop{\overset{\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\overset{|}{\mathop{C}}\,=C{{H}_{2}}}}\,}}\,\] \[+\underset{\text{Tert}\text{. butyl alcohol(side product)}}{\mathop{\underset{\,\,\,\,\,OH}{\overset{\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,}}\,+KBr+{{H}_{2}}O\]
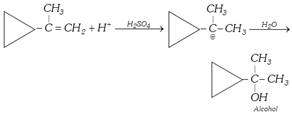
(ii) From alkenes : (a) Hydration
Direct process :
![]()
Indirect process :
\[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+\underset{\text{Sulphuric acid}}{\mathop{HOS{{O}_{2}}OH}}\,\to \underset{\text{Ethyl hydrogen sulphate}}{\mathop{C{{H}_{3}}C{{H}_{2}}OS{{O}_{2}}OH}}\,\underset{\text{Boil}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,+{{H}_{2}}S{{O}_{4}}\] \[\underset{\text{Boil}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,+{{H}_{2}}S{{O}_{4}}\]
In case of unsymmetrical alkenes
\[\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,+HOS{{O}_{2}}OH\underset{\text{rule}}{\mathop{\xrightarrow{\text{Markownikoff }\!\!'\!\!\text{ }\!\!'\!\!\text{ s}}}}\,\]
\[\underset{\,\,\,\,\,\,\,\,\,\,\,OS{{O}_{2}}OH}{\mathop{C{{H}_{3}}-\underset{|\,\,\,\,\,}{\mathop{CH}}\,-C{{H}_{3}}}}\,\underset{\text{Boil}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Propan-2-ol}}{\mathop{C{{H}_{3}}-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}}}\,\]
(b) Oxymercuration-demercuration
![]()
\[\underset{OH\,}{\mathop{-\ \underset{|}{\overset{|}{\mathop{C}}}\,\ -}}\,\underset{HgOAc}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-\ \ \ \ \ }}\,\underset{\text{Demercuration}}{\mathop{\xrightarrow{NaB{{H}_{4}}}}}\,\underset{\text{Alcohol}}{\mathop{-\underset{OH\,H}{\mathop{\underset{|}{\mathop{C}}\,\ -\underset{|}{\mathop{C}}\,-}}\,}}\,\]
This reaction is very fast and produces the alcohol in high yield. The alcohol obtained corresponds to Markownikoff’s addition of water to alkene.
(c) Hydroboration oxidation (HBO) : (Antimarkownikoff’s orientation)
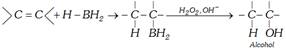
Diborane is an electron defficient molecule. It acts as an electrophile reacting with alkenes to form alkyl boranes \[{{R}_{3}}B\].
\[R-CH=C{{H}_{2}}+H-B{{H}_{2}}\to \underset{\text{Alkyl borane}}{\mathop{R-\underset{H\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-\underset{B\,{{H}_{2}}\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,}}\,\xrightarrow{RCH=C{{H}_{2}}}\]
\[\underset{\text{Dialkyl borane}}{\mathop{{{(R\,C{{H}_{2}}\,C{{H}_{2}})}_{2}}}}\,BH\xrightarrow{RCH=C{{H}_{2}}}\underset{\text{Trialkyl borane}}{\mathop{{{(RC{{H}_{2}}C{{H}_{2}})}_{3}}B}}\,\]
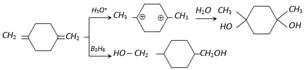
(iii) By reduction of carbonyl compounds
\[\underset{\text{Aldehyde}}{\mathop{RCHO}}\,+{{H}_{2}}\xrightarrow[LiAI{{H}_{4}}]{Pd}\underset{\text{Primary alcohol}}{\mathop{RC{{H}_{2}}OH}}\,\]
\[\underset{\text{Ketone}}{\mathop{RCO{R}'}}\,+{{H}_{2}}\underset{\text{or }Ni/Pt}{\mathop{\xrightarrow{NaB{{H}_{4}}}}}\,\underset{\text{Secondary alcohol}}{\mathop{R-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-{R}'}}\,\]
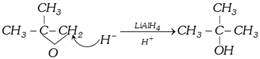
\[LiAl{{H}_{4}}\] also reduces epoxides into alcohol :
![]()
Hydride selectively attacks the less alkylated carbon of the epoxide.
(iv) By reduction of carboxylic acids and their derivatives
\[\underset{\text{Carboxylic acid}}{\mathop{R-COOH}}\,\underset{\text{(ii) }{{H}_{2}}O}{\mathop{\xrightarrow{\text{(i) }LiAl{{H}_{4}}}}}\,\underset{\text{primary alcohol}}{\mathop{RC{{H}_{2}}OH}}\,\]
\[\underset{\text{Carboxylic acid}}{\mathop{RCOOH}}\,\to \underset{\text{Ester}}{\mathop{RCOO{R}'}}\,\underset{\text{Catalyst}}{\mathop{\xrightarrow{{{H}_{2}}}}}\,RC{{H}_{2}}OH+{R}'OH\]
Esters are also reduced to alcohols
(Bouveault Blanc reaction)
\[\underset{\begin{smallmatrix}\text{Methyl acetate} \\\text{ (Ester)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-OC{{H}_{3}}}}\,\,+4[H]\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\,+\,\underset{\text{Methanol}}{\mathop{C{{H}_{3}}OH}}\,\]
\[M{{e}_{2}}C=O+\underset{\text{Isopropyl alcohol}}{\mathop{{{(C{{H}_{3}})}_{2}}CHOH}}\,\xrightarrow{Al(OCHM{{e}_{2}})}\]
![]()
(v) By alkaline hydrolysis of ester
![]()
(vi) From primary amines
\[\underset{\text{Aminoethane}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,+HONO\xrightarrow{NaN{{O}_{2}}/HCl}\]
\[\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\,\,+{{N}_{2}}+{{H}_{2}}O\]
(vii) From Grignard reagent
(a) With oxygen :
\[2R-Mg-X+{{O}_{2}}\underset{A{{l}_{2}}{{O}_{3}}}{\mathop{\xrightarrow{\Delta }}}\,2R-O-Mg-X\]
\[\xrightarrow{2HOH}2ROH+2Mg(X)OH\]
(b) With ethylene oxide :
![]()
\[RC{{H}_{2}}C{{H}_{2}}-OMgX\xrightarrow{{{H}_{2}}O}RC{{H}_{2}}C{{H}_{2}}OH+Mg(X)OH\]
(c) With carbonyl compounds :
\[\overset{\delta -}{\mathop{R}}\,-\overset{\delta +\,\,}{\mathop{Mg}}\,-X+{R}'-\underset{{{O}_{\delta -}}}{\overset{H\,\,\,\,\,\,}{\mathop{\underset{||\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,\,}{\mathop{{{C}^{\delta +}}}}}\,\,}}}\,\to \underset{\,\,\,\,\,\,\,\,\,\,OMgX}{\mathop{{R}'-\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-R\,\,}}\,\xrightarrow{{{H}_{2}}O}\underset{\,\,\,\,\,\,OH}{\mathop{{R}'-\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-R}}\,\]
(viii) The oxo process : It is also called carbonylation or hydroformylation reaction. A mixture of alkene carbon monoxides and hydrogen. Under pressure and elevated temperature in the presence of catalyst forms aldehyde.
Catalyst is cobalt carbonyl hydride \[[CoH{{(CO)}_{4}}]\] product is a mixture of isomeric straight chain (major) and branched chain (minor) aldehydes. Aldehydes are reduced catalytically to the corresponding alcohols.

![]()
(2) Physical properties of monohydric alcohols
(i) Character : Alcohols are neutral substances. These have no effect on litmus paper. This is analytical test for alcohols.
(ii) Physical state : The lower alcohols (upto \[{{C}_{12}}\]) are colourless alcohol with characteristic smell and burning taste. The higher members with more than 12-carbon atoms are colourless and odourless solids.
(iii) Polar character : Oxygen atom of the \[-OH\] group is more electronegative than both carbon and hydrogen. Thus the electron density near oxygen atom is slightly higher. Hydrogen bonding shown below
\[H-\underset{R}{\mathop{\underset{|}{\mathop{O}}\,}}\,\text{----}H-\underset{R}{\mathop{\underset{|}{\mathop{O}}\,}}\,\text{---}H-\underset{R}{\mathop{\underset{|}{\mathop{O}}\,}}\,\text{----}H-\underset{R}{\mathop{\underset{|}{\mathop{O}}\,}}\,\]. This gives polar character to OH bond.
(iv) Solubility : The lower alcohols are miscible in water.
\[H-\underset{R}{\mathop{\underset{|}{\mathop{O}}\,}}\,{{:}^{\delta -}}\text{----}{{\text{-}}^{\delta +}}H-\underset{H}{\mathop{\underset{|}{\mathop{O}}\,}}\,:\] \[\text{Solubility}\propto \frac{\text{1}}{\text{Size of alkyl groups}}\]
Increase in carbon-chain increases organic part hence solubility in water decreases.
Isomeric \[{{1}^{o}},\,{{2}^{o}},\,{{3}^{o}}\] alcohols have solubility in order \[{{1}^{o}}>\,{{2}^{o}}>\,{{3}^{o}}\].
(v) Boiling points : Due to intermolecular hydrogen bonding boiling points of alcohols are higher than hydrocarbon and ethers.
\[\text{B}\text{.P}\text{.}\propto \frac{\text{1}}{\text{No}\text{. of branches}}\]; B.P. follows the trends :
\[{{1}^{o}}\]alcohol > \[{{2}^{o}}\] alcohol > \[{{3}^{o}}\]alcohol
(vi) Density : Alcohols are lighter than water.
Density \[\propto \] Molecular masses.
(vii) In toxicating effects : Methanol is poisonous and is not good for drinking purposes. It may cause blindness and even death. Ethanol is used for drinking purposes.
(3) Chemical properties : Characteristic reaction of alcohol are the reaction of the \[-OH\] group. The reactions of the hydroxyl group consists of either cleavage of \[C-O\] bond or the cleavage of \[O-H\] bond.
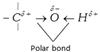
\[C-O\] bond is weaker in the case of tertiary alcohols due to \[+I\] effect of alkyl groups while \[-OH\] bond is weaker in primary alcohols as electron density increase between \[O-H\]bond and hydrogen tends to separates as a proton.

Thus primary alcohols give the most of reaction by cleavage of \[O-H\] bond while tertiary alcohols are most reactive because of cleavage of \[C-O\] bond. Hence \[-O-H\] cleavage reactivity order : Primary > Secondary > Tertiary and \[C-O-\] cleavage reactivity order : Tertiary > Secondary > Primary alcohol
(i) Reaction involving cleavage of with removal of ‘H’ as proton
Alcohols are stronger acids than terminal acetylene but are not acidic enough to react with aqueous NaOH or KOH. Acidic nature is in the order \[HOH>ROH>CH\equiv CH>N{{H}_{3}}>RH\].
Acidic nature of alcohol decrease with increase of alkyl groups on – OH bonded carbon due to +I (inductive) effect of alkyl group.
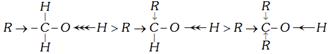
(a) Reaction with Na : (Active metals)
\[2RO-H+2M\to 2ROM+{{H}_{2}}\](M = Na, K, Mg, Al, etc.)
Evolution of \[{{H}_{2}}\] shows the presence of –OH and reaction show that alcohols are acidic in nature. Alcohols acts as Bronsted acids because they donate a proton to a strong base \[(:{{B}^{-}})\].
Example :
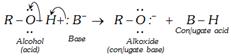
On reaction of alkoxide with water, starting alcohol is obtained.
\[\underset{\text{Acid}}{\mathop{H-\underset{.\,.}{\overset{.\,.}{\mathop{O}}}\,-H}}\,\,+\underset{\text{Base}}{\mathop{R\underset{.\,.}{\overset{.\,.}{\mathop{{{O}^{-}}}}}\,:}}\,\to \underset{\text{Conjugate acid}}{\mathop{R-O-H}}\,\,\,\,\,\,\,+\underset{\text{Conjugate base}}{\mathop{O{{H}^{-}}}}\,\]
This is the analytical test for alcohols.
(b) Reaction with carboxylic acid [Esterification] :
![]()
When HCl gas is used as catalyst, the reaction is called fischer-speier esterification.
Presence of bulky group in alcohol or in acid decreases the rate of esterification. This is due to steric hindrance of bulky group. Reactivity of alcohol in this reaction is \[{{1}^{o}}>{{2}^{o}}>{{3}^{o}}\].
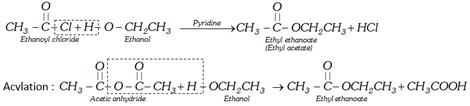
(c) Reaction with acid derivatives : (Analytical test of alcohol)
(d) Reaction with grignard reagents :
\[\underset{\begin{smallmatrix}\text{Methyl} \\\text{alcohol}\end{smallmatrix}}{\mathop{C{{H}_{3}}OH}}\,+\underset{\begin{smallmatrix}\text{Ethyl magnesium } \\\text{ bromide}\end{smallmatrix}}{\mathop{{{C}_{2}}{{H}_{5}}MgBr}}\,\to \underset{\text{Ethane}}{\mathop{{{C}_{2}}{{H}_{6}}}}\,+C{{H}_{3}}OMgBr\]
(e) Reaction with ketene :
\[R-\overset{\delta -}{\mathop{O}}\,-\overset{\delta +}{\mathop{H}}\,+C{{H}_{2}}=\overset{\delta +}{\mathop{C}}\,=\overset{\delta -}{\mathop{O}}\,\to \underset{\text{(enol form)}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,H-O}{\mathop{C{{H}_{2}}=\underset{|}{\mathop{C}}\,}}\,}}\,-O-R\to \underset{\text{(Keto form)}}{\mathop{C{{H}_{3}}-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-O-R}}\,\]
![]()
(f) Reaction with isocyanic acid :
\[R-\overset{\delta -}{\mathop{O}}\,-\overset{\delta +}{\mathop{H}}\,+H-N=\underset{{{O}_{\delta -}}\ \ \ \ \ \ \ \,}{\mathop{\underset{||}{\overset{\delta +}{\mathop{C}}}\,\to H-}}\,N=\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,\,-}}\,O-R\]
\[\to \underset{\begin{smallmatrix}\text{amino ester} \\\text{(Urethane)}\end{smallmatrix}}{\mathop{H-NH-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-O-R}}\,\]
(g) Reaction with ethylene oxide :
![]()
(h) Reaction with diazomethane :
\[R-OH+C{{H}_{2}}{{N}_{2}}\to \underset{\text{(Ether)}}{\mathop{R-O-C{{H}_{3}}+{{N}_{2}}}}\,\]
(ii) Alkylation : \[ROH+{{{R}'}_{2}}S{{O}_{4}}\to RO{R}'+{R}'HS{{O}_{4}}\]
(iii) Reaction involving cleavage of \[-\underset{|}{\overset{|}{\mathop{C}}}\,\underset{|}{\overset{|}{\mathop{-}}}\,OH\] with removal or substitution of –OH group
(a) Reaction with hydrogen halides : Alcohols give alkyl halide. The reactivity of HX is in the order of HI > HBr > HCl and the reactivity of ROH is in the order of benzyl > allyl > \[{{3}^{o}}\] > \[{{2}^{o}}\]> \[{{1}^{o}}\]. The reaction follows a nucleophilic substitution mechanism.
Grove’s process
\[ROH+HX\underset{\text{anhydrous }\Delta }{\mathop{\xrightarrow{ZnC{{l}_{2}}}}}\,R-X+{{H}_{2}}O\]
If alcohols react with HI and red phosphorus, alkane will be formed.
\[{{C}_{2}}{{H}_{5}}OH+2HI\underset{\text{heat}}{\mathop{\xrightarrow{\text{Red }P}}}\,{{C}_{2}}{{H}_{6}}+{{I}_{2}}+{{H}_{2}}O\]
Primary alcohols follow \[{{S}_{{{N}^{2}}}}\] mechanism .
\[\underset{\begin{smallmatrix}\text{Protonated} \\{{\text{1}}^{\text{o}}}\text{ alcohol}\end{smallmatrix}}{\mathop{R-OH_{2}^{+}}}\,+{{X}^{-}}\to {{\,}^{\delta -}}X\text{---}R\text{---}OH_{\text{2}}^{\delta +}\to R-X+{{H}_{2}}O\]
In secondary and tertiary alcohols, the \[{{S}_{{{N}^{1}}}}\]mechanism operates
![]()
(b) Reaction with PCl5 : \[ROH+P{{X}_{5}}\to RX+PO{{X}_{3}}+HX\]; X = Cl
(Analytical test for alcohols)
(c) Reaction with PCl3 :
\[\underset{\text{Alcohol}}{\mathop{3ROH}}\,\,\,\,\,+\underset{\begin{smallmatrix}\text{Phosphorus} \\\text{ trichloride}\end{smallmatrix}}{\mathop{PC{{l}_{3}}}}\,\to \,\,\underset{\begin{smallmatrix}\text{ Alkyl} \\\text{chloride}\end{smallmatrix}}{\mathop{3RCl}}\,+\underset{\begin{smallmatrix}\text{Phosphorus} \\\text{ acid}\end{smallmatrix}}{\mathop{{{H}_{3}}P{{O}_{3}}}}\,\]
(d) Reaction with thionyl chloride \[[SOC{{l}_{2}}]\]:
\[ROH+SOC{{l}_{2}}\xrightarrow{\text{Pyridine}}RCl+S{{O}_{2}}+HCl\]
(e) Reaction with ammonia :
\[ROH+N{{H}_{3}}\underset{{{360}^{o}}C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,\underset{\begin{smallmatrix}\text{Primary}\\\text{amine}\end{smallmatrix}}{\mathop{RN{{H}_{2}}}}\,\]\[\underset{A{{l}_{2}}{{O}_{3}}}{\mathop{\xrightarrow{ROH}}}\,\underset{\begin{smallmatrix}\text{Secondary}\\\text{amine}\end{smallmatrix}}{\mathop{{{R}_{2}}NH}}\,\underset{A{{l}_{2}}{{O}_{3}}}{\mathop{\xrightarrow{ROH}}}\,\underset{\begin{smallmatrix}\text{Tertiary}\\\text{amine}\end{smallmatrix}}{\mathop{{{R}_{3}}N}}\,\]
(f) Reaction with \[HN{{O}_{3}}\]:
![]()
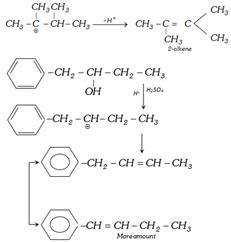
(g) Reaction with \[{{H}_{2}}S{{O}_{4}}\] [Dehydration of alcohol] : The elimination of water from a compound is known as dehydration. The order of ease dehydration is Tertiary > Secondary > primary alcohol. The products of dehydration of alcohols are depend upon the nature of dehydrating agents and temperature.
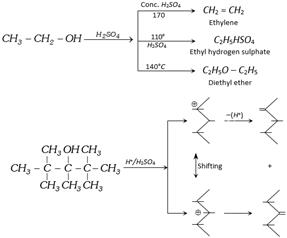
Alcohol leading to conjugated alkene are dehydrated to a greater extent than those of alcohols leading to nonconjugated alkene. Thus dehydration is in order
\[\underset{\,\,\,\,\,\,\,\,\,\,\,OH}{\mathop{C{{H}_{2}}=CH-\underset{|}{\mathop{C}}\,H-C{{H}_{3}}}}\,>\underset{\,\,\,\,\,\,\,\,\,\,\,OH}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{C{{H}_{3}}-C{{H}_{2}}-CH-C{{H}_{3}}}}\,}}\,\]
\[\underset{\,C{{H}_{3}}\,OH\,}{\overset{C{{H}_{3}}\,\,\,\,\,}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,\,-\underset{|\,\,\,\,\,}{\mathop{CH}}\,-C{{H}_{3}}}}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,\underset{\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-\underset{\oplus \,\,\,\,\,\,}{\mathop{CH}}\,}}}\,-C{{H}_{3}}\to \]
(iv) General reaction of alcohols
(a) Reduction : \[R-OH+2HI\xrightarrow{\Delta }R-H\]
(b) Oxidation : Difference between \[{{1}^{o}},\,\,{{2}^{o}}\] and \[{{3}^{o}}\]alcohols.
\[{{1}^{o}}\]\[\to\]\[RC{{H}_{2}}OH\to\underset{\text{Aldehyde}}{\mathop{R-\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,=O}}\,\to\underset{\text{Carboxylicacid}}{\mathop{R-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,=}}\,O}}\,\].
\[{{2}^{o}}\to\]\[\underset{\text{Secondary}\,\text{alcohol}}{\mathop{R-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-{R}'}}\,\xrightarrow{Cr{{O}_{3}}}R-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-{R}'\underset{\text{Drasticconditions}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,O\,\,\,\,\,\,\,}}}\,RCOOH+C{{O}_{2}}+{{H}_{2}}O\]\[\underset{\text{Drastic conditions}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,O\,\,\,\,\,\,\,}}}\,RCOOH+C{{O}_{2}}+{{H}_{2}}O\]
\[{{3}^{o}}\to \]\[\underset{\begin{smallmatrix}\text{Tert}\text{. butyl alcohol} \\\text{ (Tertiary)}\end{smallmatrix}}{\mathop{\underset{\,\,\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-OH}}}\,}}\,\underset{\begin{smallmatrix}(\text{Under strong } \\\text{ condition})\end{smallmatrix}}{\mathop{\xrightarrow{\,\,4[O]\,\,}}}\,\underset{\begin{smallmatrix}\text{ Acetone} \\\text{(Lesser number }\\\text{of carbon atoms)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{C{{H}_{3}}\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,=O}}\,}}\,\underset{\begin{smallmatrix}(\text{Under strong } \\\text{ condition})\end{smallmatrix}}{\mathop{\xrightarrow{\,\,4[O]\,\,}}}\,\underset{\begin{smallmatrix}\text{ Acetic acid} \\\text{(Lesser number}\\\text{of carbon atoms)}\end{smallmatrix}}{\mathop{C{{H}_{3}}COOH}}\,+C{{O}_{2}}+{{H}_{2}}O\]
\[\underset{\begin{smallmatrix}(\text{Under strong } \\\text{ condition})\end{smallmatrix}}{\mathop{\xrightarrow{\,\,4[O]\,\,}}}\,\underset{\begin{smallmatrix}\text{ Acetic acid} \\ \text{(Lesser number}\\\text{of carbon atoms)}\end{smallmatrix}}{\mathop{C{{H}_{3}}COOH}}\,+C{{O}_{2}}+{{H}_{2}}O\]
(c) Catalytic oxidation/dehydrogenation
\[{{1}^{o}}\]\[\underset{\begin{smallmatrix}\text{ Ethanol} \\\text{(Pri}\text{. alcohol)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-O\,H}}\,\xrightarrow{Cu,\,573K}\underset{\begin{smallmatrix}\text{Ethanal} \\\text{(Acetaldehyde)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=O}}\,+{{H}_{2}}\]
\[{{2}^{o}}\]\[\underset{\begin{smallmatrix}\text{2-Propanol} \\\text{(Sec}\text{. alcohol)}\end{smallmatrix}}{\overset{\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-O\,H}}}\,\xrightarrow{Cu,\,573K}\underset{\begin{smallmatrix}\text{Propanone}\\\text{(Acetone)}\end{smallmatrix}}{\mathop{\overset{C{{H}_{3}}}{\mathop{C{{H}_{3}}-\overset{|}{\mathop{C}}\,=O+{{H}_{2}}}}\,}}\,\]
\[{{3}^{o}}\]\[\underset{\begin{smallmatrix}\text{2-Methylpropan-2-ol} \\\text{ (Tert}\text{. alcohol)}\end{smallmatrix}}{\mathop{\underset{\,\,\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-OH}}}\,}}\,\xrightarrow{Cu,\,573K}\underset{\begin{smallmatrix}\text{2-Methylpropene} \\\text{ (Alkene)}\end{smallmatrix}}{\mathop{\overset{\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\overset{|}{\mathop{C}}\,=C{{H}_{2}}}}\,}}\,+{{H}_{2}}O\]
Important reagents used for oxidation of alcohols
(d) Self condensation : Guerbet’s reaction
\[R-C{{H}_{2}}-C{{H}_{2}}-OH+H-\underset{R\,\,\,}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{2}}-OH\]
\[\xrightarrow{NaO{{C}_{2}}{{H}_{5}},\,\Delta }\underset{\text{higher alcohol}}{\mathop{R-C{{H}_{2}}-C{{H}_{2}}-\overset{R\,\,\,}{\mathop{\overset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{2}}-OH}}\,\]
(e) Reaction with cerric ammonium nitrate : \[\underset{\text{Yellow colour}}{\mathop{\text{Cerric ammonium nitrate}}}\,\,+ROH\to \] Red colour solution of complex. This is analytical test for alcohols.
(f) Iodoform test : When a few drops of alcohol are warmed with iodine and NaOH yellow precipitate of iodoform with characteristic smell is obtained. Any alcohol consists \[C{{H}_{3}}CHOH\] group give iodoform test.
Since reaction takes place with alkali solution as one of the reagents hence alkyl halide like \[C{{H}_{3}}-C{{H}_{2}}Cl\] and \[C{{H}_{3}}-\underset{Cl\,\,}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-R\] will also give this test.
(4) Uses of monohydric alcohol :
(i) Uses of ethanol : It is used
(a) In alcoholic beverages, (b) As a solvent in paints, varnishes, oils, perfumes etc., (c) In the preparation of chemical like chloroform, ether etc., (d) As a fuel in spirit lamps, (e) As an antifreeze for automobile radiators, (f) In the scientific apparatus like spirit levels, (g) As power alcohol.
(ii) Uses of methanol :
(a) Methanol is an important industrial starting material for preparing formaldehyde, acetic acid and other chemicals.
(b) As a fuel (a petrol substitute). A 20% mixture of methyl alcohol and gasoline is a good motor fuel.
(c) As an antifreeze or automobile radiators.
(d) To denature ethyl alcohol. The mixture is called methylated spirit.
(e) In the preparation of dyes, medicines and perfumes. Methyl salicylate and methyl anthra anilate are used in perfumery.
Difference between methanol and ethanol
| Methanol | Ethanol |
| (i) When \[C{{H}_{3}}OH\] is heated on Cu coil it gives formalin like smell. | (i) It does not give formalin like smell. |
| (ii) When \[C{{H}_{3}}OH\] is heated with salicylic acid in \[{{H}_{2}}S{{O}_{4}}\] (conc.) then methyl salicylate is formed which has odour like winter green oil. | (ii) No such odour is given. |
| (iii) It does not give haloform or iodoform test. | (iii) It gives haloform test |
Interconversion of monohydric alcohols
(i) Primary alcohol into secondary alcohols
\[\underset{\begin{smallmatrix}\text{Propan-1-ol}\\\text{(1}{}^\circ\text{alcohol)}\end{smallmatrix}}{\mathop{{{C}_{3}}{{H}_{7}}OH}}\,\xrightarrow{SOC{{l}_{2}}}{{C}_{3}}{{H}_{7}}Cl\xrightarrow{\text{alc}KOH}\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,\]\[\xrightarrow{HBr}C{{H}_{3}}\underset{Br\,}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,C{{H}_{3}}\xrightarrow{\text{aq}\text{.}KOH}\underset{\begin{smallmatrix}\text{Propan-2-ol}\\\text{(2}{}^\circ\text{alcohol)}\end{smallmatrix}}{\mathop{C{{H}_{3}}\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,C{{H}_{3}}}}\,\]
(ii) Secondary alcohol into tertiary alcohol
\[\underset{\begin{smallmatrix}\text{Propan-2-ol} \\\text{(Iso-propyl alcohol)}\\\text{(2}{}^\circ \text{ alcohol)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{OH}{\mathop{\overset{|\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}}}\,\underset{{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{+}}}{\mathop{\xrightarrow{[O]}}}\,C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\]\[\xrightarrow{C{{H}_{3}}MgBr}\underset{\,\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,OMgBr}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-{{H}_{3}}}}}\,\xrightarrow{{{H}^{+}},\,{{H}_{2}}O}\underset{\begin{smallmatrix}\text{2-Methylpropan-2-ol} \\\text{(3}{}^\circ \text{) (tert}\text{. butyl alcohol)}\end{smallmatrix}}{\mathop{\underset{\,\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,OH}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,}}\,\]
(iii) Primary alcohol into tertiary alcohol
\[\underset{\begin{smallmatrix}\text{2-Methylpropan-1-ol (1}{}^\circ \text{)} \\\text{ (Iso butyl alcohol)}\end{smallmatrix}}{\mathop{\overset{\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}\overset{|\,\,\,\,\,}{\mathop{CH}}\,C{{H}_{2}}}}\,OH}}\,\underset{\text{Dehydration}}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}},\text{Heat}}}}\,\overset{\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\overset{|}{\mathop{C}}\,=C{{H}_{2}}}}\,\underset{\begin{smallmatrix}\text{Markownikoff }\!\!'\!\!\text{ s} \\\text{ rule}\end{smallmatrix}}{\mathop{\xrightarrow{\,\,\,HBr\,\,\,\,}}}\,\]
\[\underset{\,\,\,Br}{\overset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,\xrightarrow{\text{aq}\text{.}KOH}\underset{\begin{smallmatrix}\text{2-Methylpropan-2-ol (3}{}^\circ \text{)} \\\text{(tert}\text{. butyl alcohol)}\end{smallmatrix}}{\mathop{\underset{\,\,\,\,OH}{\overset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,}}\,\]
(iv) Lower alcohol into higher alcohol (ascent of series)
\[\underset{\begin{smallmatrix}\text{ Methanol} \\\text{(1 carbon atom)}\end{smallmatrix}}{\mathop{C{{H}_{3}}OH}}\,\xrightarrow{HI}C{{H}_{3}}I\xrightarrow{KCN}C{{H}_{3}}CN\]\[\underset{\text{Reduction}}{\mathop{\xrightarrow{4(H)}}}\,C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\xrightarrow{HONO}\underset{\begin{smallmatrix}\text{ Ethanol} \\\text{(2 carbon atoms)}\end{smallmatrix}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\]
(v) Higher alcohol into lower alcohol [Descent series]
\[\underset{\begin{smallmatrix}\text{ Ethanol} \\\text{(2 carbon atoms)}\end{smallmatrix}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\underset{[O]}{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}},\,{{H}^{+}}}}}\,C{{H}_{3}}COOH\xrightarrow{NaOH}C{{H}_{3}}COONa\]
\[\underset{\text{Heat}}{\mathop{\xrightarrow{NaOH+CaO}}}\,C{{H}_{4}}\xrightarrow{C{{l}_{2}}}C{{H}_{3}}Cl\xrightarrow{\text{aq}\text{. }KOH}\underset{\begin{smallmatrix}\text{ Methanol} \\\text{(one carbon atom)}\end{smallmatrix}}{\mathop{C{{H}_{3}}OH}}\,\]
Distinguish between primary, secondary and tertiary monohydric alcohols
(i) Lucas test : A mixture of anhydrous \[ZnC{{l}_{2}}+\text{conc}\text{. }HCl\] is called as Lucas reagent.
| Primary | \[R-C{{H}_{2}}-OH\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\text{conc}\text{. }HCl/ZnC{{l}_{2}}\text{ anhy}\text{.}}}}\,R-C{{H}_{2}}-Cl\to \] ppt. appears after heating |
| Secondary | \[{{R}_{2}}CH-OH\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\text{conc}\text{. }HCl/ZnC{{l}_{2}}\text{ anhy}\text{.}}}}\,{{R}_{2}}-CH-Cl\to \] ppt. appears with in 5 minutes |
| Tertiary | \[{{R}_{3}}C-OH\xrightarrow{ZnC{{l}_{2}}/HCl}{{R}_{3}}C-Cl\to \] ppt. appears immediately |
(ii) Victor mayer test : Also known as RBW test. RBW ® Red, Blue, White test.
| Primary | \[{{C}_{2}}{{H}_{5}}OH\xrightarrow{P+{{I}_{2}}}{{C}_{2}}{{H}_{5}}I\xrightarrow{AgN{{O}_{2}}}{{C}_{2}}{{H}_{5}}N{{O}_{2}}\xrightarrow{HONO}\underset{\text{Nitrolic acid}}{\mathop{C{{H}_{3}}-\underset{NOH\,\,\,\,\,\,}{\mathop{\underset{||}{\mathop{C}}\,-N{{O}_{2}}}}\,}}\,\xrightarrow{NaOH}\underset{\text{Sod}\text{. salt of nitrolic acid (Red colour)}}{\mathop{C{{H}_{3}}-\underset{NONa\,\,\,\,}{\mathop{\underset{||}{\mathop{C}}\,-N{{O}_{2}}}}\,}}\,\] |
| Secondary | \[{{(C{{H}_{3}})}_{2}}CHOH\xrightarrow{P+{{I}_{2}}}{{(C{{H}_{3}})}_{2}}CHI\xrightarrow{AgN{{O}_{2}}}{{(C{{H}_{3}})}_{2}}-\underset{H\ \ \,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,N{{O}_{2}}}}\,\xrightarrow{HONO}{{(C{{H}_{3}})}_{2}}-\underset{NO}{\mathop{\underset{|}{\mathop{C}}\,N}}\,{{O}_{2}}\xrightarrow{NaOH}\]No reaction (Blue colour) |
| Tertiary | \[{{(C{{H}_{3}})}_{3}}COH\xrightarrow{P+{{I}_{2}}}{{(C{{H}_{3}})}_{3}}Cl\xrightarrow{AgN{{O}_{2}}}{{(C{{H}_{3}})}_{3}}CN{{O}_{2}}\xrightarrow{HONO}\] No reaction (colourless) |
You need to login to perform this action.
You will be redirected in
3 sec
