Amino acids
Category : JEE Main & Advanced
Proteins are a class of biologically important compounds. They are crucial to virtually all processes in living systems. Some of them are hormones which serve as chemical messengers that coordinate certain biochemical activities. Some proteins serve to transport the substances through the organism. Proteins are also found in toxins (poisonous materials) as well as in antibiotics. All the proteins are made up of many amino acid units linked together into a long chain. An amino acid is a bifunctional organic molecule that contains both a carboxyl group, \[COOH\], as well as an amine group, \[N{{H}_{2}}\].
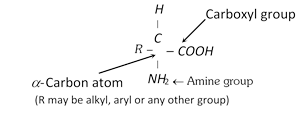
The proteins differ in the nature of R-group bonded to \[\alpha -\]carbon atom. The nature of R-group determines the properties of proteins. There are about 20 amino acids which make up the bio-proteins. Out of these 10 amino acids (non-essential) are synthesised by our bodies and rest are essential in the diet (essential amino acids) and supplied to our bodies by food which we take because they cannot be synthesised in the body. The \[\alpha -\]amino acids are classified into the following four types and tasulabed as under,
| Amino acids with non polar side chain : | |
| Name / Structure | Three letter symbol/One letter code |
|
Glycine :
|
Gly / G |
|
Alanine :
|
Ala / A |
|
Valine :
|
Val / V |
|
Leucine :
|
Leu / L |
|
Isoleucine :
|
Ile / I |
|
Phenyl alanine :
|
Phe / F |
|
Proline :
|
Pro / P |
| Amino acids with polar but neutral side chain : | |
| Name / Structure | Three letter symbol / One letter code |
|
Tryptophan :
|
Trp / W |
|
Serine :
|
Ser / S |
|
Threonine :
|
Thr / T |
|
Tyrosine :
|
Tyr / Y |
|
Cysteine :
|
Cys / C |
|
Methionine :
|
Met / M |
|
Aspargine :
|
Asn / N |
|
Glutamine :
|
Gln / Q |
|
Amino acids with acidic side chains : |
|
|
Aspartic acid :
|
Asp / D |
|
Glutamic acid :
|
Glu / E |
|
Amino acids with basic side chains : |
|
|
Lysine :
|
Lys / K |
|
Arginine :
|
Arg / R |
|
Histidine :
|
His / H |
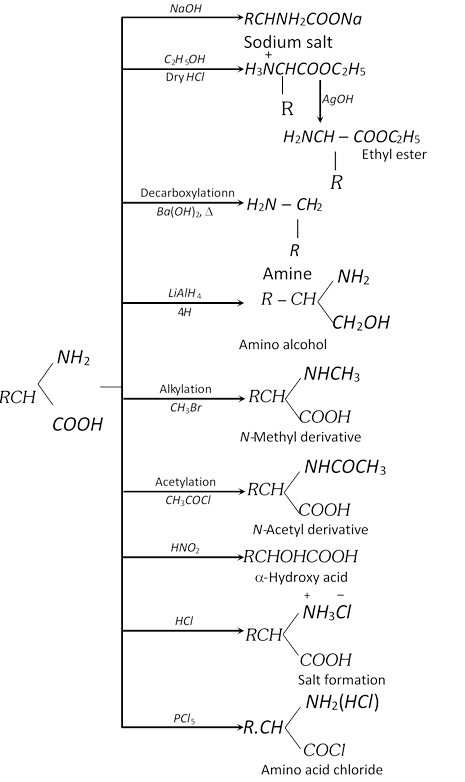
(1) Methods of preparation of a-amino acids
(i) Amination of \[\alpha -\]halo acids
\[\underset{\alpha \text{-Bromo propionic acid}}{\mathop{C{{H}_{3}}\underset{Br\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,COOH+2N{{H}_{3}}}}\,\to \underset{\begin{smallmatrix} \alpha \text{-Amino propionic acid}\,\,\,\,\,\, \\ \text{ (Alanine)} \end{smallmatrix}} {\mathop{C{{H}_{3}}\underset{N{{H}_{2}}\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,HCOOH}}\,+N{{H}_{4}}Cl}}\,\]
Lab preparation of glycine
\[\underset{\alpha \text{-Chloro acetic acid}}{\mathop{Cl.C{{H}_{2}}COOH}}\,+\underset{\text{liquid}}{\mathop{3N{{H}_{3}}}}\,\xrightarrow{50{}^\circ C}\underset{\text{Amm}\text{. salt of glycine}}{\mathop{{{H}_{2}}N.C{{H}_{2}}COON{{H}_{4}}}}\,+N{{H}_{4}}Cl\]
The ammonium salt so obtained is boiled with copper carbonate and cooled when blue colour needles of copper salt of glycine are obtained.
\[2[{{H}_{2}}N-C{{H}_{2}}COON{{H}_{4}}]+CuC{{O}_{3}}\xrightarrow{\text{Boiled}}\]\[\underset{\text{Copper salt of glycine}}{\mathop{{{({{H}_{2}}NC{{H}_{2}}COO)}_{2}}Cu}}\,+{{(N{{H}_{4}})}_{2}}C{{O}_{3}}\]
It is now dissolved in water and \[{{H}_{2}}S\] is passed till whole of the copper precipitates as copper sulphide leaving glycine as the aqueous solution.
\[{{({{H}_{2}}N-C{{H}_{2}}COO)}_{2}}Cu+{{H}_{2}}S\to \underset{\text{Glycine}}{\mathop{2{{H}_{2}}NC{{H}_{2}}COOH}}\,+\underset{\text{Black ppt}\text{.}}{\mathop{CuS}}\,\downarrow \]
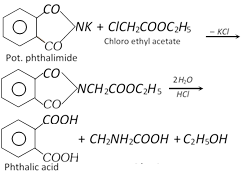
(ii) Gabriel pthalimide synthesis
(iii) Knoop synthesis
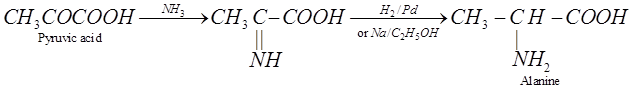
(iv) Streker synthesis

(v) From natural protein : Natural proteins are hydrolysed with dil. HCl or \[{{H}_{2}}S{{O}_{4}}\] at \[250{}^\circ C\] in an autoclave when a mixture of \[\alpha -\]amino acids is obtained. This mixture is esterified and the various esters are separated by fractional distillation. The esters are then hydrolysed into respective a-amino acids.
(2) Physical properties
(i) Amino acids are colourless, crystalline substances having sweet taste. They melt with decomposition at higher temperature (more than \[{{200}^{o}}C\]). They are soluble in water but insoluble in organic solvents.
(ii) Except glycine, all the \[\alpha -\]amino acids are optically active and have an asymmetric carbon atom (a-carbon atom). Hence, each of these amino acids can exist in two optical isomers. In proteins, however, only one isomer of each is commonly involved.
(iii) Zwitter ion and isoelectric point : Since the \[-N{{H}_{2}}\] group is basic and \[COOH\] group is acidic, in neutral solution it exists in an internal ionic form called a Zwitter ion where the proton of \[COOH\] group is transferred to the \[-N{{H}_{2}}\] group to form inner salt, also known as dipolar ion.
\[\underset{\alpha \text{-Amino acid}}{\mathop{{{H}_{2}}\overset{\bullet \,\bullet }{\mathop{N}}\,-\overset{R}{\mathop{\overset{|}{\mathop{C}}\,}}\,HCOOH}}\,\xrightarrow{\text{In water}}\] \[{{H}_{2}}\overset{\bullet \,\bullet }{\mathop{N}}\,-\overset{R}{\mathop{\overset{|}{\mathop{C}}\,}}\,H-CO\overset{-}{\mathop{O}}\,\,+\overset{+}{\mathop{H}}\,\to \underset{\begin{smallmatrix} \text{ Zwitter ion} \\ \text{(Dipolar ion)} \end{smallmatrix}}{\mathop{{{H}_{3}}\overset{+}{\mathop{N}}\,-\overset{R}{\mathop{\overset{|}{\mathop{C}}\,}}\,H-CO\overset{-}{\mathop{O}}\,}}\,\]
The Zwitter ion is dipolar, charged but overall electrically neutral and contains both a positive and negative charge.
(3) Chemical properties : Amino acids are amphoteric in nature. Depending on the pH of the solution, the amino acid can donate or accept proton.
\[\underset{\text{(Neutral not isolated)}}{\mathop{R-\underset{N{{H}_{2}}}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-OH}}\,\underset{\text{(Cation in fairly acidic medium)}}{\mathop{\underset{pH=0}{\mathop{R-\underset{\overset{\oplus }{\mathop{N}}\,{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-OH}}\,}}\,\]\[\underset{\text{(Zwitter ion in neutral medium)}}{\mathop{\underset{pH=7}{\mathop{R-\underset{\overset{\oplus }{\mathop{N}}\,{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-{{O}^{-}}}}\,}}\,\underset{\text{(Anion in fairly basic solution)}}{\mathop{\underset{pH=11}{\mathop{\underset{N{{H}_{2}}}{\mathop{R-\underset{|}{\mathop{C}}\,H}}\,\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,O}}\,}}\,\]
When an ionised form of amino acid is placed in an electric field, it will migrate towards the opposite electrode. Depending on the pH of the medium, following three things may happen
The intermediate pH at which the amino acid shows no tendency to migrate towards any of the electrodes and exists the equilibrium when placed in an electric field is known as isoelectric point.
This is characteristic of a given amino acid and depends on the nature of R-linked to a-carbon atom.
(i) Action of heat
(a) For \[\alpha -\]amino acids
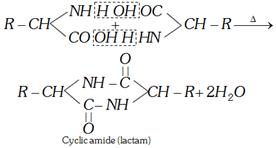
(b) For \[\beta -\]amino acids
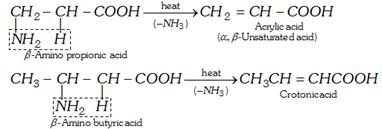
(c) For \[\gamma \] and \[\delta \] amino acids

These lactams have stable five or six membered rings.
(ii) \[\alpha -\]amino acids show the reactions of \[-N{{H}_{2}}\] group, \[-COOH\] groups and in which both the groups are involved.
(iii) Formation of proteins-peptide bond : Proteins are formed by joining the carboxyl group of one amino acid to the \[\alpha -\]amino group of another amino acid. The bond formed between two amino acids by the elimination of a water molecule is called a peptide linkage or bond. The peptide bond is simply another name for amide bond.
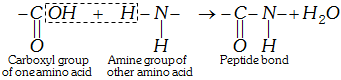
The product formed by linking amino acid molecules through peptide linkages, \[-CO-NH-\], is called a peptide. Peptides are further designated as di, tri, tetra or penta peptides accordingly as they contain two, three, four or five amino acid molecules, same or different, joined together in the following fashions.
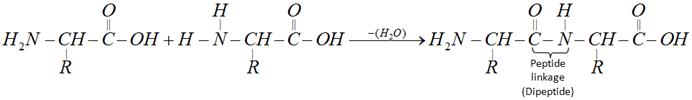
When the number of amino molecules is large, the product is termed polypeptide which may be represented as,
When the number of amino molecules is large, the product is termed polypeptide which may be represented as,
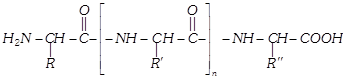
(4) Composition : Composition of a protein varies with source. An approximate composition is as follows :
Carbon 50-53%; hydrogen 6-7%; oxygen 23-25%; nitrogen 16-17%; Sulphur about 1%. Other elements may also be present, e.g., phosphorus (in nucleoproteins), iodine (in thyroid proteins) and iron (in haemoglobin).
(5) Structure of proteins : The structure of proteins is very complex. The primary structure of a protein refers to the number and sequence of the amino acids in its polypeptide chains (discussed in the formation of proteins). The primary structure is represented beginning with the amino acid whose amino group is free (the N-terminal end) and it forms the one end of the chain. Free carboxyl group (C-terminal end) forms the other end of the chain.
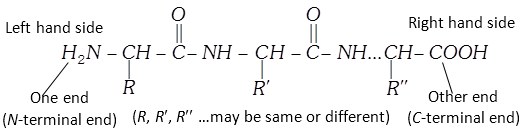
Side chains may have basic groups or acidic groups as \[-N{{H}_{2}}\] in lysine and \[COOH\] in aspartic acid. Because of these acidic and basic side chains, there are positively and negatively charged centres. Though the peptide linkage is stable, the reactivity is due to these charged centres in the side chains.
Primary structure tells us nothing about the shape or conformation of the molecule. Most of the bonds in protein molecules being single bonds can assume infinite number of shapes due to free rotation about single bonds. However, it has been confirmed that each protein has only a single three dimensional conformation. The fixed configuration of a polypeptide skeleton is referred to as the secondary structure of a protein. It gives information :
Secondary structure of protein is mainly of two types
(i) \[\alpha -\]helix : This structure is formed when the chain of a-amino acids coils as a right handed screw (called \[\alpha -\]helix) because of the formation of hydrogen bonds between amide groups of the same peptide chain, i.e., NH group in one unit is linked to carbonyl oxygen of the third unit by hydrogen bonding. This hydrogen bonding between different units is responsible for holding helix in a position. The side chains of these units project outward from the coiled backbone.
Such proteins are elastic, i.e., they can be stretched. On stretching weak hydrogen bonds break up and the peptide chain acts like a spring. The hydrogen bonds are reformed on releasing the tension. Wool and hair have \[\alpha -\]helix structure.
(ii) \[\beta -\]pleated sheet : A different type of secondary structure is possible when polypeptide chains are arranged side by side. The chains are held together by a very large number of hydrogen bonds between \[C=O\] and NH of different chains. Thus, the chains are bonded together forming a sheet. These sheets can slide over each other to form a three dimensional structure called a beta pleated sheet. Silk has a beta pleated structure.
Globular proteins possess tertiary structure. In general globular proteins are very tightly folded into a compact spherical form.
(6) Classification of proteins : According to chemical composition, proteins are divided into two classes
(i) Simple proteins : Simple proteins are composed of chains of amino acid units only joined by peptide linkages. These proteins on hydrolysis yield only mixture of amino acids. Examples are :
Egg albumin, serum globulins, glutenin in wheat, coryzenin in rice, tissue globulin, etc.
(ii) Conjugated proteins : The molecules of conjugated proteins are composed of simple proteins and non protein material. The non-protein material is called prosthetic group or cofactor. These proteins on hydrolysis yield amino acids and non-protein material. Examples are
Mucin in saliva (prosthetic group, carbohydrate), casein in milk (prosthetic group, phosphoric acid), haemoglobin in blood (prosthetic group, iron pigment), etc.
According to molecular shape, proteins are divided into two types
(i) Fibrous proteins : These are made up of polypeptide chains that run parallel to the axis and are held together by strong hydrogen and disulphide bonds. They can be stretched and contracted like a thread. These are usually insoluble in water. Examples are : \[\alpha -\]keratin (hair, wool, silk and nails); myosin (muscles); collagen (tendons, bones), etc.
(ii) Globular proteins : These have more or less spherical shape (compact structure). \[\alpha -\]helics are tightly held up by weak attractive forces of various types: Hydrogen bonding, disulphide bridges, ionic or salt bridges. These are usually soluble in water. Examples are: Insulin, pepsin, haemoglobin, cytochromes, albumins, etc.
Proteins can also be classified on the basis of their function
| Protein | Function | Examples |
| Enzymes | Biological catalysts, vital to all living systems. | Trypsin, pepsin. |
| Structural proteins | Proteins that hold living systems together. | Collagen. |
| Harmones | Act as messengers. | Insulin. |
| Transport proteins | Carry ions or molecules from place to another in the living system. | Haemoglobin. |
| Protective proteins (antibiotics) | Destroy any foreign substance released into the living system. | Gamma globulin. |
| Toxins | Poisonous in nature. | Snake venom. |
(i) Most of them (except chromoproteins) are colourless, tasteless, and odourless. Many are amorphous but few are crystalline. They are nonvolatile and do not have a sharp melting point .
(ii) Most of them are insoluble in water and alcohol. But many of them dissolve in salt solutions, dilute acids and alkalies. Some proteins such as keratins (skin, hair and nails) are completely insoluble.
(iii) Protein molecules are very complex and possess very high molecular masses. They are hydrophilic colloids which cannot pass through vegetable or animal membrane. On addition of sodium chloride, ammonium sulphate magnesium sulphate, etc., some proteins are precipitated. The precipitate can be filtered and redissolved in water.
(iv) The solution of proteins are optically active. Most of them are laevorotatory. The optical activity is due to the presence of asymmetric carbon atoms in the constituent \[\alpha -\]amino acids.
(v) Isoelectric point : Every protein has a characteristic isoelectric point at which its ionisation is minimum. Like amino acids, proteins, having charged groups (\[\overset{+}{\mathop{N}}\,{{H}_{3}}\] and \[CO{{O}^{-}}\]) at the ends of the peptide chain, are amphoteric in nature. In strong acid solution, protein molecule accepts a proton while in strong basic solution it loses a proton. The pH at which the protein molecule has no net charge is called its isoelectric point. This property can be used to separate proteins from mixture by electrophoresis.
(vi) Denaturation : The structure of the natural proteins is responsible for their biological activity. These structures are maintained by various attractive forces between different parts of the polypeptide chains. The breaking of these forces by a physical or a chemical change makes the proteins to lose all or part of their biological activity. This is called denaturation of proteins. The denaturing of proteins can be done by adding chemicals such as acids, bases, organic solvents, heavy metal ions, or urea. It can also be done with the help of heat and ultraviolet light. Denaturation can be irreversible or reversible. In irreversible denaturation, the denaturated protein does not return to its original shape. For example, the heating of white of an egg (water soluble) gives a hard and rubbery insoluble mass.
(8) Chemical properties
(i) Salt formation : Due to presence of both \[-N{{H}_{2}}\] and \[COOH\] groups in proteins, they form salts with acids and bases. Casein is present in milk as calcium salt.
(ii) Hydrolysis : The simple proteins are hydrolysed by acids, alkalies or enzymes to produce amino acids. Following steps are involved in the hydrolysis and the final product is a mixture of amino acids.
Protein \[\to \] Proteose \[\to \] Peptone \[\to \] Polypeptide \[\to \] Simple peptide \[\to \] Mixture of amino acids
(iii) Oxidation : Proteins are oxidised on burning and putrefaction. The products include amines, nitrogen, carbon dioxide and water. The bad smell from decaying dead animals is largely due to the formation of amines by bacterial oxidation of body proteins.
(i) Biuret test : On adding a dilute solution of copper sulphate to alkaline solution of protein, a violet colour is developed. This test is due to the presence of peptide \[(CONH)\] linkage.
(ii) Xanthoproteic test : Some proteins give yellow colour with concentrated nitric acid (formation of yellow stains on fingers while working with nitric acid in laboratory). The formation of yellow colour is due to reaction of nitric acid with benzenoid structures. Thus, when a protein solution is warmed with nitric acid a yellow colour may be developed which turns orange on addition of \[N{{H}_{4}}OH\] solution.
(iii) Millon’s test : When millon’s reagent (mercurous and mercuric nitrate in nitric acid) is added to a protein solution, a white precipitate which turns brick red on heating, may be formed. This test is given by proteins which yield tyrosine on hydrolysis. This is due to presence of phenolic group.
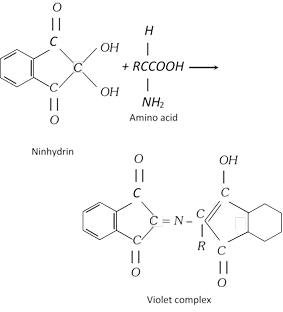
(iv) Ninhydrin test : This test is given by all proteins. When a protein is boiled with a dilute solution of ninhydrin, a violet colour is produced.
(v) Nitroprusside test : Proteins containing \[-SH\] group give this test. When sodium nitroprusside solution is added to proteins having \[-SH\] group, a violet colour is developed.
(vi) Molisch’s test : This test is given by those proteins which contain carbohydrate residue. On adding a few drops of alcoholic solution of \[\alpha -\]naphthol and concentrated sulphuric acid to the protein solution, a violet ring is formed.
(vii) Hopkins-Cole test : On adding concentrated sulphuric acid down the side containing a solution of protein and glyoxalic acid, a violet colour is developed.
(10) Uses
(i) Proteins constitute as essential part of our food. Meat, eggs, fish, cheese provide proteins to human beings.
(ii) In textile : Casein (a milk protein) is used in the manufacture of artificial wool and silk.
(iii) In the manufacture of amino acids : Amino acids, needed for medicinal use and feeding experiments, are prepared by hydrolysis of proteins.
(iv) In industry : Gelatin (protein) is used in food products, capsules and photographic plates. Glue (protein) is used as adhesive and in sizing paper. Leather is obtained by tanning the proteins of animal hides.
(v) In controlling body processes : Haemoglobin present in blood is responsible for carrying oxygen and carbon dioxide. Harmones (proteins) control various body processes.
(vi) As enzymes : Reactions in living systems always occur with the aid of substances called enzymes. Enzymes are proteins produced by living systems and catalyse specific biological reactions.
Important enzymes tabulated as under,
| Enzymes | Reaction catalysed |
| Urease | Urea \[\to C{{O}_{2}}+N{{H}_{3}}\] |
| Invertase | Sucrose \[\to \] Glucose + Fructose |
| Maltase | Maltose \[\to \] 2 Glucose |
| Amylase | Starch \[\to \] n Glucose |
| Pepsin | Proteins \[\to \] Amino acids |
| Trypsin | Proteins \[\to \] Amino acids |
| Carbonic anhydrase | \[{{H}_{2}}C{{O}_{3}}\to {{H}_{2}}O+C{{O}_{2}}\] |
| Nuclease | DNA, RNA \[\to \] Nucleotides |
You need to login to perform this action.
You will be redirected in
3 sec
