Isomerism In Co-ordination Compounds
Category : JEE Main & Advanced
Compounds having the same molecular formula but different structures or spatial arrangements are called isomers and the phenomenon is referred as isomerism.
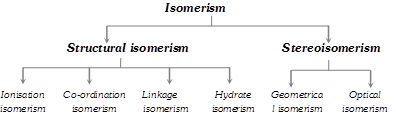
(1) Structural isomerism : Here the isomers have different arrangement of ligands around the central metal atom. It is of the following types :
(i) Ionisation isomerism : The co-ordination compound having the same composition or molecular formula but gives different ions in solution are called ionization isomers.
There is exchange of anions between the co-ordination sphere and ionization sphere.
| Example : \[[Co\,Br\,{{(N{{H}_{3}})}_{5}}]\,S{{O}_{4}}\] | \[[Co\,S{{O}_{4}}{{(N{{H}_{3}})}_{5}}]\,\,Br\] |
| Pentaaminebromo cobalt (III) Sulphate | Pentaaminesulphato cobalt (III) bromide |
| \[SO_{4}^{2-}\]present in ionisation sphere | \[B{{r}^{-}}\] present in ionization sphere |
| Gives white precipitate with \[BaC{{l}_{2}}\] | Gives light yellow precipitate with \[AgN{{O}_{3}}\] |
(ii) Co-ordination isomerism : In this case compound is made up of cation and anion and the isomerism arises due to interchange of ligands between complex cation and complex anion.
Example :
\[[Co{{(N{{H}_{3}})}_{6}}]\,\,[Cr\,{{(CN)}_{6}}]\]
\[[Cr\,{{(N{{H}_{3}})}_{6}}]\,[Co{{(CN)}_{6}}]\]
hexaamine cobalt (III) hexacyano chromate (III) hexaamine chromium (III) hexacyanocobalt (III)
complex cation contains \[\to \]\[N{{H}_{3}}\] ligand (with cobalt)
complex anion contains \[\to \]\[N{{H}_{3}}\] ligand (with chromium)
complex anion contains \[\to \]\[C{{N}^{-}}\]ligand (with chromium)
complex anion contains \[\to \]\[C{{N}^{-}}\] ligand (with cobalt)
(iii) Linkage isomerism : In this case isomers differ in the mode of attachment of ligand to central metal ion and the phenomenon is called linkage isomerism.
Example :
\[[Co\,ONO\,{{(N{{H}_{3}})}_{5}}]C{{l}_{2}}\]; \[[Co\,N{{O}_{2}}\,{{(N{{H}_{3}})}_{5}}]C{{l}_{2}}\]
Pentaamminenitritocobalt (III)
Pentaaminenitrocobalt (III) chloride
\[:O-N{{O}^{-}}\] oxygen atom donates lone pair of electrons (nitrito) \[NO_{2}^{-}\] nitrogen atom donates lone pair of electrons (nitro)
(iv) Hydrate isomerism : Hydrate isomers have the same composition but differ in the number of water molecules present as ligands and the phenomenon is called hydrate isomerism.
Examples :
(a) \[[Cr\,{{({{H}_{2}}O)}_{6}}]\,C{{l}_{3}}\] hexaaquachromium (III) chloride (violet)
(b)\[[Cr\,{{({{H}_{2}}O)}_{5}}\,Cl\,]\,C{{l}_{2}}.\,{{H}_{2}}O\] pentaaquachlorochromium (III) chloride monohydrate (blue green)
(c) \[[Cr{{({{H}_{2}}O)}_{4}}Cl]C{{l}_{2}}.2{{H}_{2}}O\] tetraaquadichloro chromium (III) chloride dihydrate (green)
(2) Stereo isomerism or space isomerism : Here the isomers differ only in the spatial arrangement of atoms of groups about the central metal atom. It is of two types :
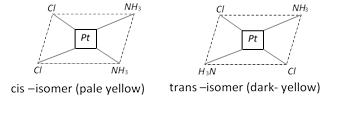
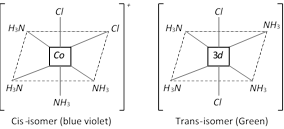
(i) Geometrical or Cis-trans isomerism : This isomerism arises due to the difference in geometrical arrangement of the ligands around the central atom. When identical ligands occupy positions near to each other called cis-isomer. When identical ligands occupy positions opposite to each other called trans –isomer. It is very common in disubstituted complexes with co-ordination number of 4 and 6.
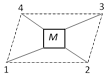
Tetrahedral geometry : In this case all the four ligands are symmetrically arranged with respect to one another as such geometrical isomerism is not possible.
Square planar geometry : The four ligands occupy position at the four corners and the metal atom or ion is at the center and lie in the same plane.
Type : I \[[M{{a}_{2}}{{b}_{2}}]\], \[M=Pt,\,a=Cl,\,b=N{{H}_{3}}\]
Example :\[\]\[[Pt\,Cl\,(N{{H}_{3}}){{(Py)}_{2}}]\]
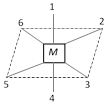
Complexes of co-ordination number 6
Octahedral geometry : Here the metal atom or ion lies at the center and 1 to 6 position are occupied by the ligands.
Cis–Positions : 1–2, 2–3, 3–4, 4–5
Trans – position : 1–4, 2–5, 3–6
Type –I \[M{{a}_{4}}{{b}_{2}}\], \[M=Co,\,a=N{{H}_{3}},\,\]and \[b=Cl\]
Example : \[[CoC{{l}_{2}}(\]\[N{{H}_{3}}{{)}_{4}}{{]}^{+}}\]ion
Type –II \[[M{{a}_{3}}{{b}_{3}}]\],\[M=Rh,\,a=Cl,\] and \[b=Py\]
Example : \[[Rh\,C{{l}_{3}}{{(Py)}_{3}}\]
Type –III \[{{[M{{(aa)}_{2}}{{(en)}_{2}}]}^{++}}\], \[M=Co,\,a-a=\underset{\text{(bidentate) }}{\mathop{\underset{C{{H}_{2}}N{{H}_{2}}\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}N{{H}_{2}}}}\,}}\,}}\,\]
\[b=Cl\] (monodentate)
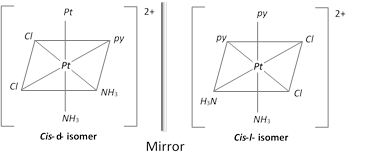
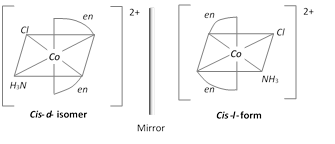
(ii) Optical isomerism
(a) Optical isomers are mirror images of each other and have chiral centers.
(b) Mirror images are not super imposable and do and have the plane of symmetry.
(c) Optical isomers have similar physical and chemical properties but differ in rotating the plane of plane polarized light.
(d) Isomer which rotates the plane polarized light to the right is called dextro rotatory (d-form) and the isomer which rotates the plane polarized light to the left is called laevorotatory (l–form)
Example : \[{{[M{{a}_{2}}{{b}_{2}}{{c}_{2}}]}^{n_{-}^{+}}};\,\,{{[Pt{{(Py)}_{2}}{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]}^{2+}}\]
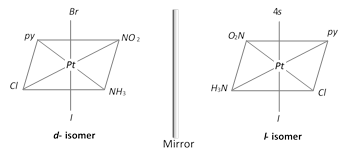
\[[M\,a\,b\,c\,d\,e\,f]\,;\,Pt\,(py)\,N{{H}_{3}}N{{O}_{2}}Cl\,Br]\]
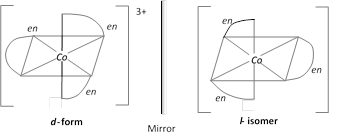
\[{{[M{{(AA)}_{3}}]}^{n\pm }};\,{{[CO{{(en)}_{3}}]}^{3+}}\]

\[{{[M{{(AA)}_{2}}{{a}_{2}}]}^{n\pm }};\,{{[Co{{(en)}_{2}}C{{l}_{2}}]}^{+}}\]

\[{{[M{{(AA)}_{2}}ab]}^{n\pm }};\,\,{{[Co{{(en)}_{2}}N{{H}_{3}}Cl]}^{2+}}\]
You need to login to perform this action.
You will be redirected in
3 sec
