Constitutional or structural isomerism
Category : JEE Main & Advanced
(1) Chain, nuclear or skeleton isomerism : This type of isomerism arises due to the difference in the nature of the carbon chain (i.e., straight or branched) which forms the nucleus of the molecule.
Examples :
(i) C4H10 \[\underset{n-\text{Butane}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}}}\,\],\[\underset{\text{Isobutane}}{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}}}\,\]
(ii) C5H12 : (Three) \[\underset{n-\text{Pentane}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}}}\,\],
\[\underset{\text{Isopentane}}{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,}{\mathop{C}}\,H}}\,-C{{H}_{2}}-C{{H}_{3}}}}\,\], \[\underset{\text{Neopentane}}{\mathop{C{{H}_{3}}\underset{C{{H}_{3}}\ \ \ \ \ }{\mathop{\overset{C{{H}_{3}}\ \ }{\mathop{\underset{\ \ |}{\mathop{\overset{\ \ \ |}{\mathop{-C}}\,}}\,-C{{H}_{3}}}}\,}}\,}}\,\]
(2) Position isomerism : It is due to the difference in the position of the substiuent atom or group or an unsaturated linkage in the same carbon chain.
(ii) C3 H6 Cl2 : \[\underset{\begin{smallmatrix} 2,2-\text{Dichloro propane,} \\ \text{(gemdihalide)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-CC{{l}_{2}}-C{{H}_{3}}}}\,\],\[\underset{\begin{smallmatrix}1,1-\text{Dichloro propane} \\\text{(gemdihalide)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-CH-C{{l}_{2}}}}\,\],
\[\underset{\text{(Vicdihalide)}}{\mathop{\underset{1,2-\text{Dichloro propane}}{\mathop{C{{H}_{3}}-\underset{Cl\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-\underset{Cl\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,}}\,}}\,\], \[\underset{\begin{smallmatrix}1,3-\text{Dichloro propane} \\ \,\,\,\,\,\,\,\,\text{(}\alpha \text{,}\gamma \text{-dihalide)}\end{smallmatrix}}{\mathop{\underset{Cl\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-C{{H}_{2}}-\underset{Cl\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,}}\,\]
(3) Functional isomerism : This type of isomerism is due to difference in the nature of functional group present in the isomers. The following pairs of compounds always form functional isomers with each other.
Examples :
(i) Alcohols and ethers (Cn H2n+2O)
C2H6O : \[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-OH}}\,\] ; \[\underset{\text{Dimethyl}\,\text{ether}}{\mathop{{{H}_{3}}C-O-C{{H}_{3}}}}\,\]
C3H8O : \[\underset{n\,-\,\text{propyl alcohol}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-OH}}\,\] ; \[\underset{\text{Ethyl methyl}\,\text{ether}}{\mathop{{{C}_{2}}{{H}_{5}}-O-C{{H}_{3}}}}\,\]
C4H10O : \[\underset{n-\text{Butyl alcohol}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-OH}}\,\]; \[\underset{\text{Diethyl ether }}{\mathop{{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}}}\,\]
(ii) Aldehydes, ketones and unsaturated alcohols …etc. (Cn H2nO)
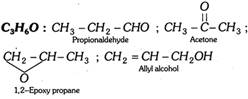
(iii) Acids, esters and hydroxy carbonyl compounds …etc. (Cn H2nO2)
C2H4O2 : \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\] ; \[\underset{\text{Methyl formate}}{\mathop{HCOOC{{H}_{3}}}}\,\]
C3H6O2 : \[\underset{\text{Propionic acid}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-COOH}}\,\] ; \[\underset{\text{Methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,\] ;
\[\underset{2-\,\text{Hydroxy propanal}}{\mathop{C{{H}_{3}}\underset{OH\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,HCHO}}\,}}\,\]; \[\underset{1-\,\text{Hydroxy propan-2-one}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-OH}}\,\]
(iv) Alkynes and alkadienes (Cn H2n-2)
C4H6 : \[\underset{1-\,\text{Butyne}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C\equiv CH}}\,\]; \[\underset{1,3-\,\text{Butadiene}}{\mathop{{{H}_{2}}C=CH-CH=C{{H}_{2}}}}\,\];
\[\underset{2-\text{Butyne}}{\mathop{C{{H}_{3}}-C\equiv C-C{{H}_{3}}}}\,\] ; \[\underset{1,2-\text{ Butadiene}}{\mathop{{{H}_{2}}C=C=CH-C{{H}_{3}}}}\,\]
(v) Nitro alkanes and alkyl nitrites (\[-N{{O}_{2}}\]and \[-O-N=O\])
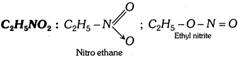
(vi) Amines (Primary, secondary and tertiary)
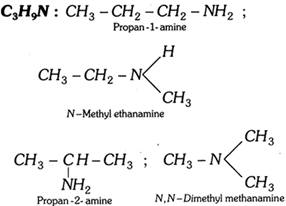
(vii) Alcohols and phenols
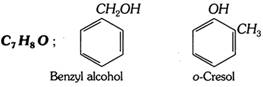
(viii) Oximes and amides
C2H5NO : \[\underset{\text{Acetaldoxime}}{\mathop{C{{H}_{3}}-CH=NOH}}\,\]; \[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,N{{H}_{2}}}}\,\]
(4) Ring-chain isomerism : This type of isomerism is due to different modes of linking of carbon atoms, i.e., the isomers possess either open chain or closed chain sturctures.
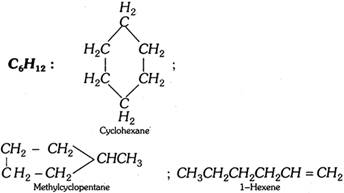
(5) Metamerism : This type of isomerism is due to the difference in the nature of alkyl groups attached to the polyvalent atoms or functional group. Metamers always belong to the same homologous series. Compounds like ethers, thio-ethers ketones, secondary amines, etc. show metamerism.
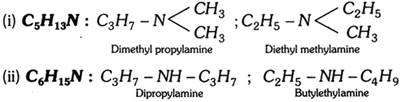
(a) \[\underset{\text{(Pentan}-\text{2}-\text{one)}}{\mathop{C{{H}_{3}}\underset{O}{\mathop{-\underset{|\,|}{\mathop{C}}\,-}}\,C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}}}\,\] ;
\[\underset{\text{(Pentan}-\text{3}-\text{one)}}{\mathop{C{{H}_{3}}C{{H}_{2}}\underset{O}{\mathop{-\underset{|\,|}{\mathop{C}}\,-}}\,C{{H}_{2}}C{{H}_{3}}}}\,\] are metamers and not position isomers.
(b) \[\underset{\text{(Pentan}-\text{2}-\text{one)}}{\mathop{C{{H}_{3}}\underset{O}{\mathop{-\underset{|\,|}{\mathop{C}}\,-}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,\] ;
\[\underset{\text{(3-Methylbutan-2-one)}}{\mathop{C{{H}_{3}}\underset{O}{\mathop{-\underset{|\,|}{\mathop{C}}\,-}}\,\underset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H-C{{H}_{3}}}}\,}}\,\] are metamers and not chain isomers.
(6) Tautomerism
(i) The type of isomerism in which a substance exist in two readily interconvertible different structures leading to dynamic equilibrium is known as tautomerism and the different forms are called tautomers (or tautomerides).
The term tautomerism (Greek: tauto = same; meros = parts) was used by Laar in 1885 to describe the phenomenon of a substance reacting chemically according to two possible structures.
(ii) It is caused by the wandering of hydrogen atom between two polyvalent atoms. It is also known as Desmotropism (Desmos = bond and tropos = turn). If the hydrogen atom oscillates between two polyvalent atoms linked together, the system is a dyad and if the hydrogen atom travels from first to third atom in a chain, the system is a triad.
(a) Dyad system : Hydrocyanic acid is an example of dyad system in which hydrogen atom oscillates between carbon and nitrogen atoms \[H-C\equiv N\rightleftharpoons C\overset{\scriptscriptstyle\leftarrow}{=}N-H\]
(b) Triad system
Keto-enol system : Polyvalent atoms are oxygen and two carbon atoms.
Examples :
\[\underset{\text{(Keto)}}{\mathop{\overset{\,\,O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,}}\,\rightleftharpoons \underset{\text{(Enol)}}{\mathop{\overset{\,\,\,\,\,OH}{\mathop{-\overset{|}{\mathop{C}}\,=}}\,\underset{|}{\mathop{C}}\,-}}\,\]
Acetoacetic ester (Ethyl acetoacetate) :
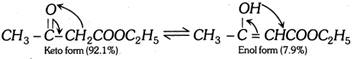
Acetoacetic ester gives certain reactions showing the presence of keto group (Reactions with \[HCN\], \[{{H}_{2}}NOH\],\[\,{{H}_{2}}NNH{{C}_{6}}{{H}_{5}}\], etc.) and certain reactions showing the presence of enolic group (Reactions with \[Na,\,C{{H}_{3}}COCl,\,N{{H}_{3}},\,PC{{l}_{5}},\,B{{r}_{2}}\]water and colour with neutral \[FeC{{l}_{3}},\] etc.). Enolisation is in order \[C{{H}_{3}}COC{{H}_{3}}<C{{H}_{3}}COC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}<{{C}_{6}}{{H}_{5}}COC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]\[<C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}\]\[<C{{H}_{3}}COC{{H}_{2}}CHO\] Acid catalysed conversion 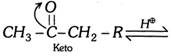 \[\underset{\text{(Intermediate)}}{\mathop{C{{H}_{3}}\overset{\,\,\,\,\,OH}{\mathop{-\underset{\oplus }{\mathop{\overset{|}{\mathop{C}}\,}}\,-}}\,\underset{H\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-R}}\,\xrightarrow{-{{H}^{\oplus }}}\underset{\text{(Enol form)}}{\mathop{C{{H}_{3}}\overset{\,\,\,\,OH}{\mathop{-\overset{|}{\mathop{C}}\,=}}\,CH-R}}\,\] Base catalysed conversion
\[\underset{\text{(Intermediate)}}{\mathop{C{{H}_{3}}\overset{\,\,\,\,\,OH}{\mathop{-\underset{\oplus }{\mathop{\overset{|}{\mathop{C}}\,}}\,-}}\,\underset{H\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-R}}\,\xrightarrow{-{{H}^{\oplus }}}\underset{\text{(Enol form)}}{\mathop{C{{H}_{3}}\overset{\,\,\,\,OH}{\mathop{-\overset{|}{\mathop{C}}\,=}}\,CH-R}}\,\] Base catalysed conversion 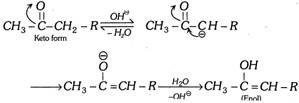 (c) Triad system containing nitrogen : Examples Nitrous acid exists in 2 forms
(c) Triad system containing nitrogen : Examples Nitrous acid exists in 2 forms 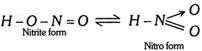 Nitro acinitro system
Nitro acinitro system 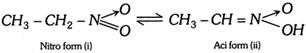 (iii) Characteristics of tautomerism (a) Tautomerism (cationotropy) is caused by the oscillation of hydrogen atom between two polyvalent atoms present in the molecule. The change is accompanied by the necessary rearrangement of single and double bonds. (b) It is a reversible intramolecular change. (c) The tautomeric forms remain in dynamic equilibrium. Hence, their separation is a bit difficult. Although their separation can be done by special methods, yet they form a separate series of stable derivatives. (d) The two tautomeric forms differ in their stability. The less stable form is called the labile form. The relative proportion of two forms varies from compound to compound and also with temperature, solvent etc. The change of one form into another is also catalysed by acids and bases. (e) Tautomers are in dynamic equilibrium with each other and interconvertible\[(\rightleftharpoons ).\] (f) Two tautomers have different functional groups. (g) Tautomerism has no effect on bond length. (h) Tautomerism has no contribution in stabilising the molecule and does not lower its energy. (i) Tautomerism may occur in planar or nonplanar molecules. r Keto=enol tautomerism is exhibited only by such aldehydes and ketones which contain at least one \[\alpha \]-hydrogen. For example \[C{{H}_{3}}CHO,\,C{{H}_{3}}C{{H}_{2}}CHO,\,C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}\] etc,. r Tautomerism is not possible in benzaldehyde \[({{C}_{6}}{{H}_{5}}CHO)\], benzophenone \[({{C}_{6}}{{H}_{5}}CO{{C}_{6}}{{H}_{5}})\], tri methyl acetaldehyde, \[{{(C{{H}_{3}})}_{3}}C-CHO\] and chloral \[CC{{l}_{3}}-CHO\] as they do not have \[\alpha -H\]. Number of structural isomers
(iii) Characteristics of tautomerism (a) Tautomerism (cationotropy) is caused by the oscillation of hydrogen atom between two polyvalent atoms present in the molecule. The change is accompanied by the necessary rearrangement of single and double bonds. (b) It is a reversible intramolecular change. (c) The tautomeric forms remain in dynamic equilibrium. Hence, their separation is a bit difficult. Although their separation can be done by special methods, yet they form a separate series of stable derivatives. (d) The two tautomeric forms differ in their stability. The less stable form is called the labile form. The relative proportion of two forms varies from compound to compound and also with temperature, solvent etc. The change of one form into another is also catalysed by acids and bases. (e) Tautomers are in dynamic equilibrium with each other and interconvertible\[(\rightleftharpoons ).\] (f) Two tautomers have different functional groups. (g) Tautomerism has no effect on bond length. (h) Tautomerism has no contribution in stabilising the molecule and does not lower its energy. (i) Tautomerism may occur in planar or nonplanar molecules. r Keto=enol tautomerism is exhibited only by such aldehydes and ketones which contain at least one \[\alpha \]-hydrogen. For example \[C{{H}_{3}}CHO,\,C{{H}_{3}}C{{H}_{2}}CHO,\,C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}\] etc,. r Tautomerism is not possible in benzaldehyde \[({{C}_{6}}{{H}_{5}}CHO)\], benzophenone \[({{C}_{6}}{{H}_{5}}CO{{C}_{6}}{{H}_{5}})\], tri methyl acetaldehyde, \[{{(C{{H}_{3}})}_{3}}C-CHO\] and chloral \[CC{{l}_{3}}-CHO\] as they do not have \[\alpha -H\]. Number of structural isomers
| Molecular formula | Number of isomers |
| Alkanes | |
| \[{{C}_{4}}{{H}_{10}}\] | Two |
| \[{{C}_{5}}{{H}_{12}}\] | Three |
| \[{{C}_{6}}{{H}_{14}}\] | Five |
| \[{{C}_{7}}{{H}_{16}}\] | Nine |
| \[{{C}_{8}}{{H}_{18}}\] | Eighteen |
| \[{{C}_{9}}{{H}_{20}}\] | Thirty five |
| \[{{C}_{10}}{{H}_{22}}\] | Seventy five |
| Alkenes and cycloalkanes | |
| \[{{C}_{3}}{{H}_{6}}\] | Two (One alkene + one cycloalkane) |
| \[{{C}_{4}}{{H}_{8}}\] | Six (Four alkene + 2 - cycloalkane) |
| \[{{C}_{5}}{{H}_{10}}\] | Nine (Five alkenes + 4 ? cycloalkanes) |
| Alkynes | |
| \[{{C}_{3}}{{H}_{4}}\] | Two |
| \[{{C}_{4}}{{H}_{6}}\] | Six |
| Monohalides | |
| \[{{C}_{3}}{{H}_{7}}X\] | Two |
| \[{{C}_{4}}{{H}_{9}}X\] | Four |
| \[{{C}_{5}}{{H}_{11}}X\] | Eight |
| Dihalides | |
| \[{{C}_{2}}{{H}_{4}}{{X}_{2}}\] | Two |
| \[{{C}_{3}}{{H}_{6}}{{X}_{2}}\] | Four |
| \[{{C}_{4}}{{H}_{8}}{{X}_{2}}\] | Nine |
| \[{{C}_{5}}{{H}_{10}}{{X}_{2}}\] | Twenty one |
| Alcohols and ethers | |
| \[{{C}_{2}}{{H}_{6}}O\] | Two (One alcohol and one ether) |
| \[{{C}_{3}}{{H}_{8}}O\] | Three (Two alcohols and one ether) |
| \[{{C}_{4}}{{H}_{10}}O\] | Seven (Four alcohols and three ethers) |
| \[{{C}_{5}}{{H}_{12}}O\] | Fourteen (Eight alcohols and six ethers) |
| Aldehydes and ketones | |
| \[{{C}_{3}}{{H}_{6}}O\] | Two (One aldehyde and one ketone) |
| \[{{C}_{4}}{{H}_{8}}O\] | Three (Two aldehydes and one ketone) |
| \[{{C}_{5}}{{H}_{10}}O\] | Seven (Four aldehydes and three ketone) |
| Monocarboxylic acids and esters | |
| \[{{C}_{2}}{{H}_{4}}{{O}_{2}}\] | Two (One acid and one ester) |
| \[{{C}_{3}}{{H}_{6}}{{O}_{2}}\] | Three (One acid and two esters) |
| \[{{C}_{4}}{{H}_{8}}{{O}_{2}}\] | Six (Two acids and four esters) |
| \[{{C}_{5}}{{H}_{10}}{{O}_{2}}\] | Thirteen (Four acids and nine esters) |
| Aliphatic amines | |
| \[{{C}_{2}}{{H}_{7}}N\] | Two (One 1°-amine and one 2°-amine) |
| \[{{C}_{3}}{{H}_{9}}N\] | Four (Two 1°-amines, one 2°-amine and one 3°-amine) |
| \[{{C}_{4}}{{H}_{11}}N\] | Eight (Four 1°-amines, three 2°-amines and one 3°-amines) |
| Aromatic compounds | |
| \[{{C}_{8}}{{H}_{10}}\] | Four |
| \[{{C}_{9}}{{H}_{12}}\] | Nine |
| \[{{C}_{7}}{{H}_{8}}O\] | Five |
You need to login to perform this action.
You will be redirected in
3 sec
