Reaction Intermediates
Category : JEE Main & Advanced
Short lived fragments called reaction intermediates result from homolytic and heterolytic bond fission. The important reaction intermediates are free radicals, carbocations, carbanions, carbenes, benzyne and nitrenes.
Negativecharge on C
| Characteristic | Free radical | Carbocation | Carbanion | Carbene |
| Nature | Neutral having odd electron | Positive charge on C
|
Negative charge on C |
Neutral, divalent with 2 unshared electrons |
| Hybridisation | sp2 | sp2 |
sp3 (non-conjugated) sp2 (Conjugated) |
(i) sp2 (singlet) (ii) sp (triplet) |
| Structure | Planar | Planar | Pyramidal/Planar |
(i) Planar (singlet) (ii) Linear (triplet) |
| Magnetism | Paramagnetic | Diamagnetic | Diamagnetic |
(i) Diamagnetic (ii) Paramagnetic |
|
Stability order |
\[P{{h}_{3}}\overset{.}{\mathop{C}}\,>P{{h}_{2}}\overset{.\,\,\,\,\,\,\,}{\mathop{CH}}\,>\] \[Ph\overset{.\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,>\] \[C{{H}_{2}}=CH-\overset{.\ \ \ \ }{\mathop{C{{H}_{2}}}}\,\] \[>{{3}^{o}}>{{2}^{o}}>\] \[{{1}^{o}}>\overset{.\ \ \ \ }{\mathop{C{{H}_{2}}}}\,>\] \[C{{H}_{2}}=\overset{.\ \ \ }{\mathop{CH}}\,\] |
\[P{{h}_{3}}\overset{+}{\mathop{C}}\,>P{{h}_{2}}\overset{+\,\,\,\,\,}{\mathop{CH}}\,>\] \[\overset{+\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{PhC{{H}_{2}}}}\,>\] \[C{{H}_{2}}=CH-\overset{+\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,>\] \[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}>\overset{+\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}}}\,\] |
\[\overset{\,\,\,\,\,\,\,}{\mathop{P{{h}_{3}}C}}\,>\overset{\,\,\,}{\mathop{P{{h}_{2}}CH}}\,>\] \[\overset{}{\mathop{PhC{{H}_{2}}}}\,>\text{Allyl}>\] \[\overset{}{\mathop{C{{H}_{2}}}}\,>{{1}^{o}}>{{2}^{o}}>{{3}^{o}}\] |
Triplet > singlet |
Benzyne
(1) 1, 2-Didehydrobenzene, \[{{C}_{6}}{{H}_{4}}\] and its derivatives are called benzyne or arynes and the simplest member is benzyne.
(2) It is neutral reaction intermediate derived from benzene ring by removing two substituents, of ortho positions, one in the form of electrophile and other in the from of nucleophile leaving behind two electrons to be distributed between two orbitals.
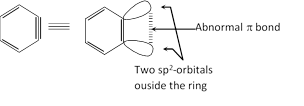
(3) Benzyne intermediate is aromatic in character.
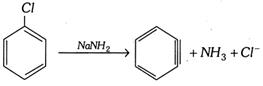
(4) When halobenzene is heated with sodamide formation of benzyne takes place.
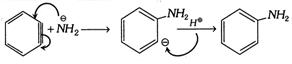
(5) (i) It behaves as dienophile and gives Diels-Alder reaction with diene.
(ii) It reacts with strong nucleophile like \[\overset{\Theta \,\,\,\,\,\,\,}{\mathop{N{{H}_{2}}}}\,\]
Nitrenes (R - N : )
(1) The nitrogen analogous of carbenes are called nitrenes.
(2) There is possibility of two spin states for nitrenes depending on whether the two non-bonding electrons (the normal nitrogen lone pair remains paired) have their spins paired or parallel.

(3) In general nitrenes obey Hunds rule and the ground state triplet with two degenerate \[sp\]-orbitals containing a single electron each.

(4) Nitrenes can be generated, in situ, by the following methods,
(i) By action of \[B{{r}_{2}}\] in presence of a base on a \[{{1}^{o}}\] amide (Hofmann-bromamide reaction),
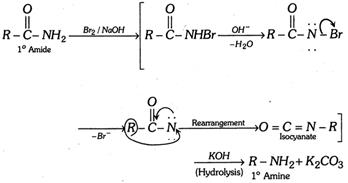
\[\underset{\text{(Hydrolysis)}}{\mathop{\xrightarrow{KOH}}}\,\,\underset{{{\text{1}}^{\text{o}}}\text{ Amine}}{\mathop{\,R-N{{H}_{2}}}}\,+{{K}_{2}}C{{O}_{3}}\]
(ii) By decomposition of azides in presence of heat or light.
\[\underset{\text{Alkyl}\,\text{azide}}{\mathop{R-\overset{.\,\,.}{\mathop{N}}\,=\overset{+}{\mathop{N}}\,=\overset{.\,\,.}{\mathop{N}}\,{{:}^{-}}}}\,\,\,\xrightarrow{\Delta \,or\,\text{h}\nu }\,\underset{\text{Alkyl}\,\text{nitrene}}{\mathop{R-\overset{.\,\,.}{\mathop{N}}\,:}}\,+N\equiv N\]
(iii) Unsubstituted nitrene \[(H-\overset{.\,\,.}{\mathop{N}}\,:)\] can be obtained by photolysis of (or by passing electric discharge through) \[N{{H}_{3}},\,{{N}_{2}}{{H}_{4}}\] or \[{{N}_{3}}H\].
You need to login to perform this action.
You will be redirected in
3 sec
