Tetra-Halides (Carbon tetrachloride, \[CC{{l}_{4}}\])
Category : JEE Main & Advanced
It is the most important tetrahalogen derivative of methane.
(1) Manufacture
(i) From methane :
\[C{{H}_{4}}+4C{{l}_{2}}\xrightarrow{400{}^\circ C}CC{{l}_{4}}+4HCl\]
(ii) From carbon disulphide :
\[C{{S}_{2}}+3C{{l}_{2}}\xrightarrow{Fe/{{I}_{2}}/AlC{{l}_{3}}}CC{{l}_{4}}+\underset{\text{monochloride}}{\mathop{\underset{\text{Sulphur}}{\mathop{{{S}_{2}}C{{l}_{2}}}}\,}}\,\]
\[{{S}_{2}}C{{l}_{2}}\] further reacts with \[C{{S}_{2}}\] to form more of carbon tetrachloride.
\[C{{S}_{2}}+2{{S}_{2}}C{{l}_{2}}\xrightarrow{{}}CC{{l}_{4}}+6S\]
Carbon tetrachloride is separated out by fractional distillation. It is washed with sodium hydroxide and then distilled to get a pure sample.
(iii) From propane :
\[{{C}_{3}}{{H}_{8}}+9C{{l}_{2}}\underset{\text{70-100}}{\mathop{\xrightarrow{\text{400}{}^\circ C}}}\,\underset{\text{(Liquid)}}{\mathop{\underset{\text{Carbon}\,\text{tetrachloride}}{\mathop{CC{{l}_{4}}}}\,}}\,+\underset{\text{(Solid)}}{\mathop{\underset{\text{Hexachloroethane}}{\mathop{{{C}_{2}}C{{l}_{6}}}}\,}}\,+8HCl\]
(2) Physical properties
(i) It is a colourless liquid having characteristic smell.
(ii) It is non-inflammable and poisonous. It has boiling point \[{{77}^{o}}C\].
(iii) It is insoluble in water but soluble in organic solvents.
(iv) It is an excellent solvent for oils, fats, waxes and greases.
(3) Chemical properties : Carbon tetrachloride is less reactive and inert to most organic reagents. However, the following reactions are observed.
(i) Reaction with steam (Oxidation) :
\[CC{{l}_{4}}+{{H}_{2}}O\xrightarrow{500{}^\circ C}\underset{\text{Phosgene (Carbonyl chloride)}}{\mathop{COC{{l}_{2}}}}\,+2HCl\]
(ii) Reduction :
\[CC{{l}_{4}}+2H\xrightarrow{Fe/{{H}_{2}}O}CHC{{l}_{3}}+HCl\]
(iii) Hydrolysis :
\[CC{{l}_{4}}+4KOH\xrightarrow{-4KCl}\underset{\text{Unstable}}{\mathop{[C{{(OH)}_{4}}]}}\,\]\[\xrightarrow{-2{{H}_{2}}O}C{{O}_{2}}\xrightarrow{2KOH}{{K}_{2}}C{{O}_{3}}+{{H}_{2}}O\]
(iv) Reaction with phenol (Reimer-tiemann reaction) :
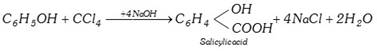
(4) Uses
(i) It is used as a fire extinguisher under the name pyrene. The dense vapours form a protective layer on the burning objects and prevent the oxygen or air to come in contact with the burning objects.
(ii) It is used as a solvent for fats, oils, waxes and greases, resins, iodine etc.
(iii) It finds use in medicine as helmenthicide for elimination of hook worms.
You need to login to perform this action.
You will be redirected in
3 sec
