Alkanes [Paraffines]
Category : JEE Main & Advanced
“Alkanes are saturated hydrocarbon containing only carbon-carbon single bond in their molecules.”
Alkanes are less reactive so called paraffins; because under normal conditions alkanes do not react with acids, bases, oxidising agents and reducing agent.
General formula : \[{{C}_{n}}{{H}_{2n+2}}\]
Examples are \[C{{H}_{4}},\,{{C}_{2}}{{H}_{6}},\,{{C}_{3}}{{H}_{8}}\],
(1) General Methods of preparation
(i) By catalytic hydrogenation of alkenes and alkynes (Sabatie and sanderen’s reaction)
\[\underset{\text{Alkene}}{\mathop{{{C}_{n}}{{H}_{2n}}}}\,+{{H}_{2}}\underset{\text{heat}}{\mathop{\xrightarrow{Ni}}}\,\underset{\text{Alkane}}{\mathop{{{C}_{n}}{{H}_{2n+2}}}}\,\]; \[\underset{Alkyne}{\mathop{{{C}_{n}}{{H}_{2n-2}}}}\,+2{{H}_{2}}\underset{\text{heat}}{\mathop{\xrightarrow{Ni}}}\,\underset{\text{Alkane}}{\mathop{{{C}_{n}}{{H}_{2n+2}}}}\,\]
(ii) Birch reduction :
\[R-CH=C{{H}_{2}}\underset{2.\,C{{H}_{3}}OH}{\mathop{\xrightarrow{1.\,Na/N{{H}_{3}}}}}\,R-C{{H}_{2}}-C{{H}_{3}}\]
(iii) From alkyl halide
(a) By reduction : \[RX+{{H}_{2}}\xrightarrow{Zn/HCl}RH+HX\]
(b) With hydrogen in presence of pt/pd : \[RX+{{H}_{2}}\xrightarrow{Pd\,orPt.}RH+HX\]
(c) With HI in presence of Red phosphorus : \[\underset{\text{Purpose of Red }P\text{ is to remove }{{I}_{\text{2}}}\text{ in the form of }P{{I}_{\text{3}}}}{\mathop{RBr+2HI\xrightarrow{{}}RH+HBr+{{I}_{2}}}}\,\]
(iv) By Zn-Cu couple :
\[2C{{H}_{3}}C{{H}_{2}}OH+\underset{\text{Zn-Cu}\,\text{couple}}{\mathop{Zn}}\,\xrightarrow{Cu}\underset{\text{Zinc}\,\text{ethoxide}}{\mathop{{{(C{{H}_{3}}C{{H}_{2}}O)}_{2}}Zn}}\,+2H\]
\[RX+2H\xrightarrow{{}}RH+HX\]
(v) Wurtz reaction :
![]()
(vi) Frankland’s reaction :
\[2RX+Zn\xrightarrow{{}}R-R+Zn{{X}_{2}}\]
(vii) Corey-house synthesis
\[C{{H}_{3}}-C{{H}_{2}}-Cl\underset{2.\,CuI}{\mathop{\xrightarrow{1.\,Li}}}\,{{(C{{H}_{3}}-C{{H}_{2}})}_{2}}LiCu\xrightarrow{C{{H}_{3}}-C{{H}_{2}}-Cl}\]\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
(viii) From Grignard reagent
(a) By action of acidic ‘H’ :
\[\underset{\text{halide}}{\mathop{\underset{\text{Alkyl magnesium}}{\mathop{RMgX}}\,}}\,+\underset{\text{Water}}{\mathop{HOH}}\,\xrightarrow{{}}\underset{\text{Alkane}}{\mathop{RH}}\,+Mg(OH)X\]
(b) By reaction with alkyl halide :
![]()
(ix) From carboxylic acids
(a) Laboratory method [Decarboxylation reaction or Duma reaction] \[R\,\,COONa+NaOH\underset{CaO}{\mathop{\xrightarrow{heat}}}\,\underset{\text{Alkane}}{\mathop{R-H}}\,+N{{a}_{2}}C{{O}_{3}}\]
(b) Kolbe’s synthesis :
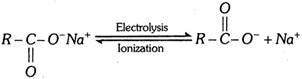
At anode [Oxidation] :
\[\underset{O\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,|\,\,\,\,\,\,\,\,\,\,\,}{\mathop{2R-C-{{O}^{-}}-2{{e}^{-}}}}\,}}\,\xrightarrow{{}}2R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-\overset{\bullet }{\mathop{O}}\,\xrightarrow{{}}2\overset{\bullet }{\mathop{R}}\,+2C{{O}_{2}}\]
\[2\overset{\bullet }{\mathop{R}}\,\xrightarrow{{}}R-R\] (alkane)
At cathode [Reduction] :
\[2N{{a}^{+}}+2{{e}^{-}}\xrightarrow{{}}2Na\xrightarrow{2{{H}_{2}}O}2NaOH+{{H}_{2}}\] \[(\uparrow )\]
(c) Reduction of carboxylic acid :
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+6HI\underset{p}{\mathop{\xrightarrow{\operatorname{Re}duction}}}\,\underset{\text{Ethane}}{\mathop{C{{H}_{3}}C{{H}_{3}}}}\,+2{{H}_{2}}O+3{{I}_{2}}\]
(x) By reduction of alcohols, aldehyde, ketones or acid derivatives
\[\underset{\text{(Methyl alcohol)}}{\mathop{\underset{\text{Methanol}}{\mathop{C{{H}_{3}}OH}}\,}}\,+2HI\underset{{{150}^{o}}C}{\mathop{\xrightarrow{\text{Red}\,P}}}\,\underset{\text{Methane}}{\mathop{C{{H}_{4}}}}\,+{{H}_{2}}O+{{I}_{2}}\]
\[\underset{\text{(Ethanal)}}{\mathop{\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,}}\,+4HI\underset{{{150}^{o}}C}{\mathop{\xrightarrow{\text{Red}\,P}}}\,\underset{\text{Ethane}}{\mathop{{{C}_{2}}{{H}_{6}}}}\,+{{H}_{2}}O+2{{I}_{2}}\]
\[\underset{\text{(Propanone)}}{\mathop{\underset{\text{Acetone}}{\mathop{C{{H}_{3}}COC{{H}_{3}}}}\,}}\,+4HI\underset{{{150}^{o}}C}{\mathop{\xrightarrow{\text{Red}\,P}}}\,\underset{\text{Propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}}}\,+{{H}_{2}}O+2{{I}_{2}}\]
\[\underset{\text{(Ethanoyl chloride)}}{\mathop{\underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-Cl}}\,}}\,+6HI\underset{{{200}^{o}}C}{\mathop{\xrightarrow{\text{Red}\,P}}}\,\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,+{{H}_{2}}O+HCl+3{{I}_{2}}\]
\[\underset{\text{(Ethanamide)}}{\mathop{\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-N{{H}_{2}}}}\,}}\,+6HI\underset{{{200}^{o}}C}{\mathop{\xrightarrow{\text{Red}\,P}}}\,\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,+{{H}_{2}}O+N{{H}_{3}}+3{{I}_{2}}\]
Aldehyde and ketones when reduced with amalgamated zinc and conc. \[HCl\] also yield alkanes.
Clemmenson reduction :
\[\underset{\text{(Ethanal)}}{\mathop{\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,}}\,+4H\underset{HCl}{\mathop{\xrightarrow{Zn-Hg}}}\,\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,+{{H}_{2}}O\]
\[\underset{\text{(Propanone)}}{\mathop{\underset{\text{Acetone}}{\mathop{C{{H}_{3}}COC{{H}_{3}}}}\,}}\,+4H\underset{HCl}{\mathop{\xrightarrow{Zn-Hg}}}\,\underset{\text{Propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}}}\,+{{H}_{2}}O\]
Wolff-kishner reduction :
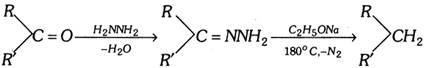
(xi) Hydroboration of alkenes
(a) On treatment with acetic acid
\[\underset{\text{Alkene}}{\mathop{R-CH=C{{H}_{2}}}}\,\xrightarrow{{{B}_{2}}{{H}_{6}}}\underset{\text{Trialkyl borane}}{\mathop{{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B}}\,\xrightarrow{C{{H}_{3}}COOH}\]\[\underset{\text{Alkane}}{\mathop{R-C{{H}_{2}}-C{{H}_{3}}}}\,\]
(b) Coupling of alkyl boranes by means of silver nitrate
\[6[R-CH=C{{H}_{2}}]\xrightarrow{2{{B}_{2}}{{H}_{6}}}{{[2R-C{{H}_{2}}-C{{H}_{2}}-]}_{3}}B\underset{NaOH}{\mathop{\xrightarrow{AgN{{O}_{3}}\,{{25}^{o}}C}}}\,\]\[3[RC{{H}_{2}}C{{H}_{2}}-C{{H}_{2}}C{{H}_{2}}R]\]
(2) Physical Properties
(i) Physical state : Alkanes are colourless, odourless and tasteless.
Alkanes State
\[{{C}_{1}}-{{C}_{4}}\] Gaseous state
\[{{C}_{5}}-{{C}_{17}}\] Liquid state [Except neo pentane which is gas]
\[{{C}_{18}}\] and above Solid like waxes
(ii) Density : Alkanes are lighter than water.
(iii) Solubility : Insoluble in water, soluble in organic solvents, solubility \[\propto \frac{1}{\text{Molecular mass}}\]
(iv) Boiling points and Melting points : Melting points and boiling points. \[\propto \] Molecular mass \[\propto \frac{1}{\text{No}\text{.}\,\text{of}\,\text{branches}}\]
| Alkane : | \[{{C}_{3}}{{H}_{8}}\] | \[{{C}_{4}}{{H}_{10}}\] | \[{{C}_{5}}{{H}_{12}}\] | \[{{C}_{6}}{{H}_{14}}\] | \[{{C}_{7}}{{H}_{16}}\] | \[{{C}_{8}}{{H}_{18}}\] |
| M.P.(K) : | 85.9 | 138 | 143.3 | 179 | 182.5 | 216.2 |
(3) Chemical properties
(i) Substitution reactions of Alkanes
(a) Halogenation : \[R-H+X-X\xrightarrow{{}}R-X+HX\]
The reactivity of halogen is : \[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{I}_{2}}\]
\[C{{H}_{4}}+2Cl-Cl\underset{-2HCl}{\mathop{\xrightarrow{u.\,v.\,light}}}\,C{{H}_{2}}-C{{l}_{2}}\underset{-HCl}{\mathop{\xrightarrow{u.\,v.\,light,\,C{{l}_{2}}}}}\,\]\[CHC{{l}_{3}}\underset{C{{l}_{2}}}{\mathop{\xrightarrow{-HCl}}}\,CC{{l}_{4}}\]
(ii) Reaction based on free radical mechanism
(a) Nitration : \[\underset{\text{Alkane}}{\mathop{R-H}}\,+HON{{O}_{2}}\underset{temp.}{\mathop{\xrightarrow{High}}}\,\underset{\text{Nitroalkane}}{\mathop{R-N{{O}_{2}}}}\,+{{H}_{2}}O\]
Nitrating mixture : (i) \[(Con.\,HN{{O}_{3}}+Con.\,{{H}_{2}}S{{O}_{4}})\] at \[{{250}^{o}}C\]
(ii) \[(HN{{O}_{3}}\,\text{vapour}\,\text{at }\,{{400}^{o}}-{{500}^{o}}C)\].
(b) Sulphonation : Free radical mechanism \[R-H+HOS{{O}_{3}}H\underset{\text{Prolonged}\,\text{heating}}{\mathop{\xrightarrow{\,\,\,\,\,\,\text{S}{{\text{O}}_{\text{3}}}\,\,\,\,}}}\,R-S{{O}_{3}}H+{{H}_{2}}O\]
(iii) Oxidation
(a) Complete Oxidation or combustion :
\[{{C}_{n}}{{H}_{2n+2}}+\left( \frac{3n+1}{2} \right){{O}_{2}}\xrightarrow{{}}nC{{O}_{2}}+(n+1){{H}_{2}}O+Q\]
(b) Incomplete combustion or oxidation
\[2C{{H}_{4}}+3{{O}_{2}}\xrightarrow{Burn}2CO+4{{H}_{2}}O\]
\[C{{H}_{4}}+\,\,{{O}_{2}}\xrightarrow{{}}C\,+2{{H}_{2}}O\]
(c) Catalytic Oxidation : \[C{{H}_{4}}+[O]\underset{100\,atm/{{200}^{o}}C}{\mathop{\xrightarrow{Cu-tube}}}\,\,C{{H}_{3}}OH\]
This is the industrial method for the manufacture of methyl alcohol.
\[C{{H}_{3}}{{(C{{H}_{2}})}_{n}}C{{H}_{3}}\underset{100-{{160}^{o}}C}{\mathop{\xrightarrow{{{O}_{2}}}}}\,C{{H}_{3}}{{(C{{H}_{2}})}_{n}}COOH\]
(d) Chemical oxidation :
\[\underset{\text{Isobutane}}{\mathop{{{(C{{H}_{3}})}_{3}}CH}}\,\xrightarrow{KMn{{O}_{4}}}\underset{\text{Tertiary butyl alcohol}}{\mathop{{{(C{{H}_{3}})}_{3}}.C.OH}}\,\]
(iv) Thermal decomposition or cracking or pyrolysis or fragmentation
\[\underset{\text{Methane}}{\mathop{C{{H}_{4}}}}\,\xrightarrow{{{1000}^{o}}C}C+2{{H}_{2}}\]
\[\underset{\text{Ethane}}{\mathop{{{C}_{2}}{{H}_{6}}}}\,\underset{C{{r}_{2}}{{O}_{3}}+A{{l}_{2}}{{O}_{3}}}{\mathop{\xrightarrow{{{500}^{o}}C}}}\,\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+{{H}_{2}}\]
\[{{C}_{3}}{{H}_{8}}\xrightarrow{{}}{{C}_{2}}{{H}_{4}}+C{{H}_{4}}\] or \[{{C}_{3}}{{H}_{6}}+{{H}_{2}}\]
(v) Isomerisation :
\[\underset{n\text{-Butane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,\underset{{{200}^{o}}C,\,35atm}{\mathop{\xrightarrow{AlC{{l}_{3}}+HCl}}}\,\underset{\text{Isobutane}}{\mathop{C{{H}_{3}}\overset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CHC{{H}_{3}}}}\,}}\,}}\,\]
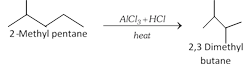
(vi) Aromatisation :
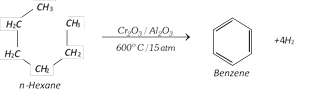
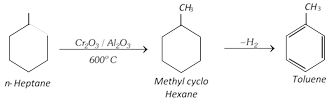
(vii) Step up reaction
(a) Reaction with \[C{{H}_{2}}{{N}_{2}}\](Diazo methane) :
\[R-C{{H}_{2}}-H+C{{H}_{2}}{{N}_{2}}\xrightarrow{hv}R-C{{H}_{2}}-C{{H}_{2}}-H\]
(b) Reaction with \[CHC{{l}_{3}}/NaOH\] :
![]()
(c) Reaction with \[C{{H}_{2}}=\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,\] :
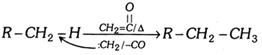
(viii) HCN formation :
\[2C{{H}_{4}}\xrightarrow{{{N}_{2}}/electric\,arc}2HCN+3{{H}_{2}}\] or
\[C{{H}_{4}}+N{{H}_{3}}\underset{{{700}^{o}}C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,HCN+3{{H}_{2}}\]
(ix) Chloro sulphonation/Reaction with SO2+Cl2
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}+S{{O}_{2}}+C{{l}_{2}}\xrightarrow{u.v\,light}\]\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}S{{O}_{2}}Cl+HCl\]
This reaction is known as reed’s reaction.
(x) Action of steam : \[C{{H}_{4}}+{{H}_{2}}O\underset{{{800}^{o}}C}{\mathop{\xrightarrow{Ni/A{{l}_{2}}{{O}_{3}}}}}\,CO+3{{H}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
