Dienes
Category : JEE Main & Advanced
These are hydrocarbon with two carbon-carbon double bonds. Dienes are of three types
(1) Conjugated dienes : Double bonds are seperated by one single bond.
Ex : \[C{{H}_{2}}=CH-CH=C{{H}_{2}}\] (1, 3-butadiene)
(2) Cumulative dienes : Double bonds are adjacent to each other.
Ex : \[C{{H}_{2}}=C=C{{H}_{2}}\] Propadiene [allene]
(3) Isolated or Non-conjugated : Double bonds are separated by more than one single bond.
Ex : \[C{{H}_{2}}=CH-C{{H}_{2}}-CH=C{{H}_{2}}\] (1, 4 pentadiene)
The general formula is \[{{C}_{n}}{{H}_{2n-2}}\]. The predominant member of this class is 1, 3-butadiene.
(1) Method of preparation
(i) From acetylene :
\[2HC\equiv CH\underset{N{{H}_{4}}Cl}{\mathop{\xrightarrow{C{{u}_{2}}C{{l}_{2}}}}}\,\underset{\text{Vinyl acetylene}}{\mathop{HC\equiv C-CH=C{{H}_{2}}}}\,\underset{Pd/BaS{{O}_{4}}}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\] \[\underset{\text{1, 3-Butadiene}}{\mathop{C{{H}_{2}}=CH-CH=C{{H}_{2}}}}\,\]
(ii) From 1, 4-dichlorobutane :
\[\underset{\text{1,4-Dichlorobutane}}{\mathop{\overset{Cl\,\,\,}{\mathop{\overset{|\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,C{{H}_{2}}C{{H}_{2}}\overset{Cl\,\,\,}{\mathop{\overset{|\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,}}\,\xrightarrow{Alc.\,KOH}\underset{\text{1, 3-Butadiene}}{\mathop{C{{H}_{2}}=CH-CH=C{{H}_{2}}}}\,\]
(iii) From 1,4-butanediol :
\[\underset{\text{1, 4-Butanediol}}{\mathop{\overset{OH}{\mathop{\overset{|\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,C{{H}_{2}}C{{H}_{2}}\overset{OH}{\mathop{\overset{|\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,}}\,\underset{\text{heat}}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,\underset{\text{1, 3-Butadiene}}{\mathop{C{{H}_{2}}=CH-CH=C{{H}_{2}}}}\,\]
(iv) From butane :
\[\underset{\text{n-Butane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,\underset{{{600}^{o}}C}{\mathop{\xrightarrow{\text{Catalyst}}}}\,\underset{\text{1, 3-Butadiene}}{\mathop{C{{H}_{2}}=CH-CH=C{{H}_{2}}}}\,\]
(\[C{{r}_{2}}{{O}_{3}}\] used as catalyst.)
(v) From cyclohexene :

(2) Physical property : 1,3-butadiene is a gas.
(3) Chemical properties
(i) Addition of halogens :

(ii) Addition of halogen acids :
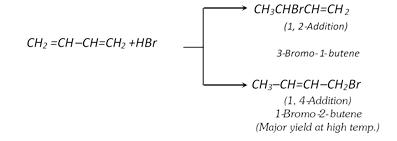
(iii) Addition of water :
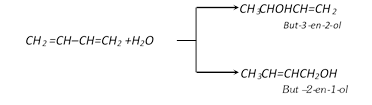
(iv) Polymerisation :
\[\underset{\text{1, 3-Butadiene}}{\mathop{nC{{H}_{2}}=CHCH=C{{H}_{2}}}}\,\xrightarrow{\text{Peroxide}}{{[-\underset{\text{Buna rubber}}{\mathop{C{{H}_{2}}CH=CHC{{H}_{2}}}}\,-]}_{n}}\]
Diels-alder reaction :
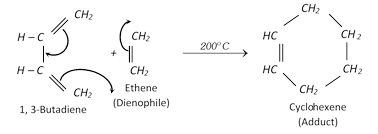
Stability of conjugated dienes : It is explained on the basis of delocalisation of electron cloud between carbon atoms.
The four \[\pi \] electrons of 1, 3-butadiene are delocalised over all the four atoms. This delocalisation of the \[\pi \] electrons makes the molecule more stable.
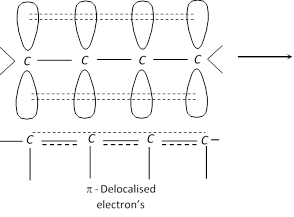
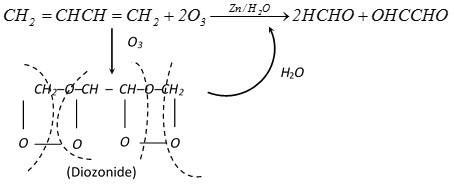
(v) Ozonolysis :
\[C{{H}_{2}}=CHCH=C{{H}_{2}}+2{{O}_{3}}\xrightarrow{Zn/{{H}_{2}}O}2HCHO+OHCCHO\]
You need to login to perform this action.
You will be redirected in
3 sec
