Alkyl nitrites and nitro alkanes
Category : JEE Main & Advanced
Nitrous acid exists in two tautomeric forms.
![]()
Corresponding to these two forms, nitrous acid gives two types of derivatives, i.e., alkyl nitrites and nitro alkanes.
![]()
It is important to note that nitro alkanes are better regarded as nitro derivatives of alkanes, while alkyl nitrites are regarded as alkyl esters of nitrous acid.
(1) Alkyl nitrites : The most important alkyl nitrite is ethyl nitrite.
Ethyl nitrite \[({{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{ONO})\]
(i) General methods of preparation : It is prepared
(a) By adding concentrated HCl or \[{{H}_{2}}S{{O}_{4}}\] to aqueous solution of sodium nitrite and ethyl alcohol at very low temperature \[(0{}^\circ C)\].
\[NaN{{O}_{2}}+\,HCl\,\to \,NaCl+HN{{O}_{2}}\]
\[{{C}_{2}}{{H}_{5}}OH+HN{{O}_{2}}\to \,\underset{\text{Ethyl nitrite}}{\mathop{{{C}_{2}}{{H}_{5}}ONO}}\,\,\ +{{H}_{2}}O\]
(b) From Ethyl iodide
\[\underset{\text{Ethyl iodide}}{\mathop{{{C}_{2}}{{H}_{5}}I}}\,+\,\underset{\text{Pot}\text{. nitrite}}{\mathop{KONO}}\,\,\to \underset{\text{Ethyl nitrite}}{\mathop{{{C}_{2}}{{H}_{5}}ONO}}\,\ +KI\]
(c) By the action of \[{{N}_{2}}{{O}_{3}}\] on ethyl alcohol.
\[2{{C}_{2}}{{H}_{5}}OH+{{N}_{2}}{{O}_{3}}\to 2{{C}_{2}}{{H}_{5}}ONO+{{H}_{2}}O\]
(ii) Physical properties
(a) At ordinary temperature it is a gas which can be liquified on cooling to a colourless liquid, (boiling point \[17{}^\circ C\]) having characteristic smell of apples.
(b) It is insoluble in water but soluble in alcohol and ether.
(iii) Chemical properties
(a) Hydrolysis : It is hydrolysed by aqueous alkalies or acids into ethyl alcohol.
\[{{C}_{2}}{{H}_{5}}ONO+{{H}_{2}}O\xrightarrow{NaOH}{{C}_{2}}{{H}_{5}}OH+HN{{O}_{2}}\]
(b) Reduction :
\[{{C}_{2}}{{H}_{5}}ONO\,+6H\underset{HCl}{\mathop{\xrightarrow{Sn}}}\,{{C}_{2}}{{H}_{5}}OH+N{{H}_{3}}+{{H}_{2}}O\]
Small amount of hydroxylamine is also formed.
\[{{C}_{2}}{{H}_{5}}ONO+4H\to {{C}_{2}}{{H}_{5}}OH+N{{H}_{2}}OH\]
(iv) Uses
(a) Ethyl nitrite dialates the blood vessels and thus accelerates pulse rate and lowers blood pressure, so it is used as a medicine for the treatment of asthma and heart diseases (angina pectoris).
(b) Its 4% alcoholic solution (known as sweet spirit of nitre) is used in medicine as a diuretic.
(c) Since it is easily hydrolysed to form nitrous acids, it is used as a source of nitrous acid in organic synthesis.
(2) Nitro alkanes or Nitroparaffins : Nitro alkanes are regarded as nitro derivatives of hydrocarbons.
(i) Classification : They are classified as primary, secondary and tertiary depending on the nature of carbon atom to which nitro groups is linked.

(ii) General methods of preparation
(a) By heating an alkyl halide with aqueous alcoholic solution of silver nitrite
\[{{C}_{2}}{{H}_{5}}Br+AgN{{O}_{2}}\to {{C}_{2}}{{H}_{5}}N{{O}_{2}}+AgBr\]
Some quantity of alkyl nitrite is also formed in this reaction. It can be removed by fractional distillation since alkyl nitrites have much lower boiling points as compared to nitro alkanes.
(b) By the direct nitration of paraffins (Vapour phase nitration)
\[C{{H}_{3}}C{{H}_{3}}+HON{{O}_{2}}(\text{fuming})\,\xrightarrow{400{}^\circ C}C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}+{{H}_{2}}O\]
With higher alkanes, a mixture of different nitro alkanes is formed which can be separated by fractional distillation.
(c) By the action of sodium nitrite on a-halo carboxylic acids
\[\underset{\alpha \text{--Chloro acetic acid}}{\mathop{C{{H}_{2}}ClOOH}}\,\underset{-NaCl}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\underset{\alpha \text{Nitro acetic acid}}{\mathop{C{{H}_{2}}N{{O}_{2}}COOH}}\,\]\[\xrightarrow{\text{heat}}\underset{\text{Nitro methane}}{\mathop{C{{H}_{3}}N{{O}_{2}}}}\,\ +C{{O}_{2}}\]
(d) By the hydrolysis of \[\alpha -\]nitro alkene with water or acid or alkali (Recent method)
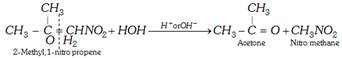
(e) Tertiary nitro alkanes are obtained by the oxidation of t-alkyl amines with \[KMn{{O}_{4}}\].
\[{{R}_{3}}CN{{H}_{2}}\xrightarrow{KMn{{O}_{4}}}{{R}_{3}}CN{{O}_{2}}+{{H}_{2}}O\]
(iii) Physical properties
(a) Nitro alkanes are colourless, pleasant smelling liquids.
(b) These are sparingly soluble in water but readily soluble in organic solvents.
(c) Their boiling points are much higher than isomeric alkyl nitrites due to polar nature.
(d) Again due to polar nature, nitro alkanes are excellent solvents for polar and ionic compounds.
\[\underset{(nitro\text{-form})}{\mathop{C{{H}_{3}}-\underset{O}{\mathop{\underset{\downarrow }{\mathop{N}}\,}}\,=O}}\,\] \[\underset{(aci\text{-form})}{\mathop{C{{H}_{2}}=\underset{O}{\mathop{\underset{\downarrow }{\mathop{N}}\,}}\,-OH}}\,\]
(iv) Chemical properties
(a) Reduction : Nitro alkanes are reduced to corresponding primary amines with Sn and HCl or Fe and HCl or catalytic hydrogenation using nickel as catalyst.
\[RN{{O}_{2}}+6H\to RN{{H}_{2}}+2{{H}_{2}}O\]
However, when reduced with a neutral reducing agent (Zinc dust\[+\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{4}}}\mathbf{Cl}\]), nitro alkanes form substituted hydroxylamines.
\[RN{{O}_{2}}+4H\xrightarrow{Zn+N{{H}_{4}}Cl}R-NHOH+{{H}_{2}}O\]
(b) Hydrolysis : Primary nitro alkanes on hydrolysis form hydroxylamine and carboxylic acid.
\[RC{{H}_{2}}N{{O}_{2}}+{{H}_{2}}O\xrightarrow{HCl\,\,\text{or}\,80%{{H}_{2}}S{{O}_{4}}}RCOOH+N{{H}_{2}}OH\]
secondary nitro alkanes on hydrolysis form ketones.
\[2{{R}_{2}}CHN{{O}_{2}}\xrightarrow{HCl}\underset{Ketone}{\mathop{2{{R}_{2}}CO}}\,\,+{{N}_{2}}O+{{H}_{2}}O\]
(c) Action of nitrous acid : Nitrous acid reacts with primary, secondary and tertiary nitro alkanes differently.
\[R-\underset{\underset{\text{Primary}}{\mathop{N{{O}_{2}}}}\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,+\underset{\text{Nitrous acid}}{\mathop{O=NOH}}\,\xrightarrow{-{{H}_{2}}O}\underset{\text{Nitrolic acid}}{\mathop{\underset{N{{O}_{2}}\,\,\,\,}{\mathop{R-\underset{|}{\mathop{C}}\,=NOH}}\,}}\,\]\[\xrightarrow{NaOH}\underset{\text{Red coloured}\,\text{sodium}\,\text{salt}}{\mathop{\underset{N{{O}_{2}}\,\,\,\,\,\,}{\mathop{R-\underset{|}{\mathop{C}}\,=NONa}}\,}}\,\]
\[\underset{\text{Secondary}\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{N{{O}_{2}}\,\,\,\,\,\,\,\,\,}{\mathop{{{R}_{2}}\underset{|}{\mathop{C}}\,H+HON}}\,}}\,=O\xrightarrow{-{{H}_{2}}O}\underset{\text{Pseudo nitrol}}{\mathop{\underset{N{{O}_{2}}}{\mathop{{{R}_{2}}\underset{|}{\mathop{C}}\,-NO}}\,}}\,\underset{NaOH}{\mathop{\xrightarrow{\text{Ether or}}}}\,\text{Blue colour}\]
Tertiary nitro alkanes do not react with nitrous acid.
(d) Thermal decomposition : .
\[R.C{{H}_{2}}.C{{H}_{2}}N{{O}_{2}}\underset{\text{moderately}}{\mathop{\xrightarrow{>300{}^\circ C}}}\,R.CH=C{{H}_{2}}+HN{{O}_{2}}\]
On rapid heating nitro alkanes decompose with great violence.
\[C{{H}_{3}}N{{O}_{2}}\xrightarrow{\text{heat, Rapidly}}\frac{1}{2}{{N}_{2}}+C{{O}_{2}}+\frac{3}{2}{{H}_{2}}\]
(e) Halogenation : Primary and secondary nitro alkanes are readily halogenated in the a-position by treatment with chlorine or bromine.
\[C{{H}_{3}}-N{{O}_{2}}\underset{NaOH}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{\text{Chloropicrin or nitro chloroform (insecticide)}}{\mathop{CC{{l}_{3}}N{{O}_{2}}}}\,\]
\[\underset{2-\text{Nitropropane}}{\mathop{C{{H}_{3}}\overset{C{{H}_{3}}\,\,}{\mathop{\overset{|}{\mathop{C}}\,H}}\,N{{O}_{2}}}}\,\xrightarrow{C{{l}_{2}}\,+\,NaOH}C{{H}_{3}}\underset{Cl\,\,\,\,\,\,\,\,}{\overset{C{{H}_{3}}\,\,\,\,}{\mathop{\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,N{{O}_{2}}}}}\,\]
(f) Condensation with aldehyde :
\[C{{H}_{3}}CHO+C{{H}_{3}}N{{O}_{2}}\to \underset{\text{(nitro alcohol)}}{\mathop{\underset{\beta \text{-Hydroxy nitropropane}}{\mathop{C{{H}_{3}}CH(OH)C{{H}_{2}}N{{O}_{2}}}}\,}}\,\]
(g) Reaction with grignard reagent : The aci-form of nitroalkane reacts with Grignard reagent forming alkane.
![]()
![]()
Thus \[{{1}^{o}}\] and \[{{2}^{o}}\] nitroalkanes are acidic mainly due to following two reasons,
(a) Strong electron withdrawing effect of the \[\text{ }N{{O}_{2}}\] group.
(b) Resonance stabilisation of the carbanion (I) formed after the removal of proton.
The aci-form of nitroalkanes is relatively more acidic because it produces relatively more conjugate base.
(v) Uses : Nitro alkanes are used,
(a) As solvents for polar substances such as cellulose acetate, synthetic rubber etc.
(b) As an explosive.
(c) For the preparation of amines, hydroxylamines, chloropicrin etc.
Distinction between Ethyl nitrite and Nitro ethane
| Test |
Ethyl nitrite \[({{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{ONO})\] (Alkyl nitrite, RONO) |
Nitro ethane \[({{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{N}{{\mathbf{O}}_{\mathbf{2}}})\] (Nitro alkane, \[\mathbf{RN}{{\mathbf{O}}_{\mathbf{2}}}\]) |
| Boiling point |
Low, \[17{}^\circ C\] |
Much higher, \[115{}^\circ C\] |
| Reduction with metal and acid (Sn/HCl) or with \[LiAl{{H}_{4}}\]. |
Gives alcohol + hydroxyl amine or \[N{{H}_{3}}\]. \[{{C}_{2}}{{H}_{5}}ONO+4H\to {{C}_{2}}{{H}_{5}}OH+N{{H}_{2}}OH\]\[RONO+6H\to ROH+N{{H}_{3}}+{{H}_{2}}O\] |
Gives corresponding primary amine. \[{{C}_{2}}{{H}_{5}}N{{O}_{2}}+6H\to {{C}_{2}}{{H}_{5}}N{{H}_{2}}+2{{H}_{2}}O\] \[RN{{O}_{2}}+6H\to RN{{H}_{2}}+2{{H}_{2}}O\] |
| Action of NaOH (alkalies). |
Readily hydrolysed to give corresponding alcohol and sodium nitrite (decomposition). \[{{C}_{2}}{{H}_{5}}ONO+NaOH\to {{C}_{2}}{{H}_{5}}OH+NaN{{O}_{2}}\] \[RONO+NaOH\to ROH+NaN{{O}_{2}}\] |
Not decomposed, i.e., alcohols are not produced. But it may form soluble sodium salt, because in presence of alkali the nitro form changes into aci form, which dissolves in alkalies to form sodium salt. |
|
Action of \[HN{{O}_{2}}\] \[(NaN{{O}_{2}}+\text{ }HCl)\] |
No action with nitrous acid. | Primary nitro alkanes forms nitrolic acid, which dissolve in alkali to give red solution. Secondary nitro alkane yields pseudo-nitrol, which dissolves in alkali to give blue solution. Tertiary nitro alkanes does not react with nitrous acid. |
You need to login to perform this action.
You will be redirected in
3 sec
