Aniline
Category : JEE Main & Advanced
Aniline was first prepared by Unverdorben (1826) by dry distillation of indigo. In the laboratory, it can be prepared by the reduction of nitrobenzene with tin and hydrochloric acid.
\[\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,+6H\xrightarrow{Sn,HCl}\underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,+2{{H}_{2}}O\]
Aniline produced combines with \[{{H}_{2}}SnC{{l}_{6}}(SnC{{l}_{4}}+2HCl)\] to form a double salt.
\[2{{C}_{6}}{{H}_{5}}N{{H}_{2}}+SnC{{l}_{4}}+2HCl\to (\underset{\text{Double salt}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{3}}{{)}_{2}}}}\,SnC{{l}_{6}}\]
From double salt, aniline is obtained by treating with conc. caustic soda solution.
\[{{({{C}_{6}}{{H}_{5}}N{{H}_{3}})}_{2}}SnC{{l}_{6}}+8NaOH\to 2{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]\[+6NaCl+N{{a}_{2}}Sn{{O}_{3}}+5{{H}_{2}}O\]
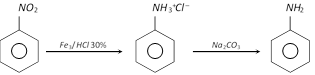
On a commercial scale, aniline is obtained by reducing nitrobenzene with iron filings and hydrochloric acid.
Aniline is also obtained on a large scale by the action of amine on chlorobenzene at \[{{200}^{o}}C\] under 300-400 atm pressure in presence of cuprous catalyst.
\[2{{C}_{6}}{{H}_{5}}Cl+2N{{H}_{3}}+C{{u}_{2}}O\underset{300-400\,\,atm}{\mathop{\xrightarrow{\ 200{}^\circ C\ }}}\,2{{C}_{6}}{{H}_{5}}N{{H}_{2}}+C{{u}_{2}}C{{l}_{2}}+{{H}_{2}}O\]
Properties Aniline when freshly prepared is a colourless oily liquid (b.p. \[{{184}^{o}}C\]). It has a characteristic unpleasant odour and is not poisonous in nature. It is heavier than water and is only slightly soluble. It is soluble in alcohol, ether and benzene. Its colour changes to dark brown on standing.
It shows all the characteristic reactions discussed earlier.
Uses : (1) It is used in the preparation of diazonium compounds which are used in dye industry.
(2) Anils (Schiff's bases from aniline) are used as antioxidants in rubber industry.
(3) It is used for the manufacture of its some derivatives such as acetamide, sulphanilic acid and sulpha drugs, etc.
(4) It is used as an accelerator in vulcanizing rubber.
You need to login to perform this action.
You will be redirected in
3 sec
