Diazonium salts
Category : JEE Main & Advanced
The diazonium salts have the general formula \[ArN_{2}^{+}{{X}^{}}\], where X– may be an anion like Cl–, Br– etc. and the group \[N_{2}^{+}(-N\equiv {{N}^{+}})\] is called diazonium ion group.
(1) Nomenclature : The diazonium salts are named by adding the word diazonium to the name of the parent aromatic compound to which they are related followed by the name of the anion. For example,
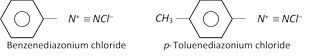
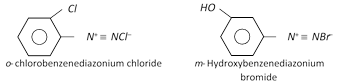
The diazonium salt may contain other anions also such as \[NO_{3}^{},HSO_{4}^{},B{{F}_{4}}\] etc.

(2) Preparation of diazonium salts :
\[NaN{{O}_{2}}+HCl\to NaCl+HONO\]
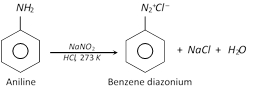
The reaction of converting aromatic primary amine to diazonium salt is called diazotisation.
(3) Physical properties of diazonium salts
(i) Diazonium salts are generally colourless, crystalline solids.
(ii) These are readily soluble in water but less soluble in alcohol.
(iii) They are unstable and explode in dry state. Therefore, they are generally used in solution state.
(iv) Their aqueous solutions are neutral to litmus and conduct electricity due to the presence of ions.
(4) Chemical properties of diazonium salts
(i) Substitution reaction : In substitution or replacement reactions, nitrogen of diazonium salts is lost as \[{{N}_{2}}\] and different groups are introduced in its place.
(a) Replacement by \[-OH\] group
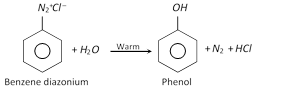
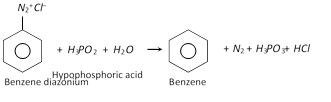
(b) Replacement by hydrogen
(c) Replacement by \[-Cl\] group
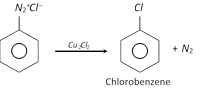
This reaction is called Sandmeyer reaction.
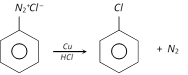
When the diazonium salt solution is warmed with copper powder and the corresponding halogen acid, the respective halogen is introduced. The reaction is a modified form of Sandmeyer reaction and is known as Gattermann reaction.
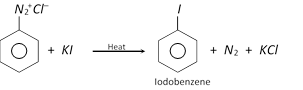
(d) Replacement by iodo \[(-I)\] group
(e) Replacement by \[-F\] group
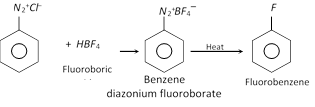
This reaction is called Balz Schiemann reaction.
(f) Replacement by Cyano \[(-CN)\]group
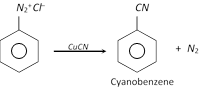
The nitriles can be hydrolysed to acids.
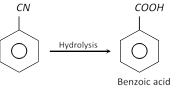
This method of preparing carboxylic acids is more useful than carbonation of Grignard reagents.
(g) Replacement by \[-N{{O}_{2}}\] group
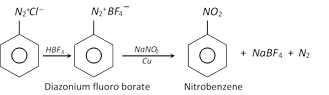
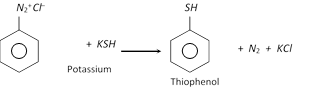
(h) Replacement by thio \[(-SH)\] group
(ii) Coupling reactions : The diazonium ion acts as an electrophile because there is positive charge on terminal nitrogen. It can react with nucleophilic aromatic compounds \[(Ar-H)\] activated by electron donating groups (\[-OH\] and \[-N{{H}_{2}}\]), which as strong nucleophiles react with aromatic diazonium salts. Therefore, benzene diazonium chloride couples with electron rich aromatic compounds like phenols and anilines to give azo compounds. The azo compounds contain \[-N=N-\] bond and the reaction is called coupling reaction.
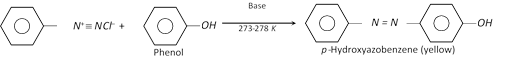
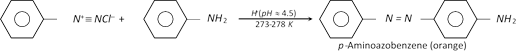
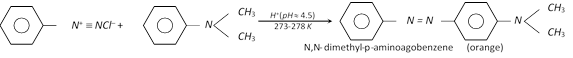
Coupling occurs para to hydroxy or amino group. All azo compounds are strongly coloured and are used as dyes. Methyl orange is an important dye obtained by coupling the diazonium salt of sulphanilic acid with N, N-dimethylaniline.
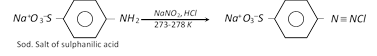
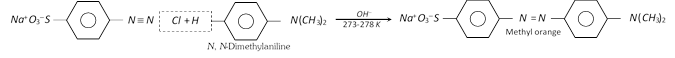
(5) Uses of diazonium salts
(i) For the manufacture of azo dyes.
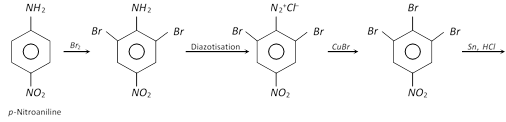
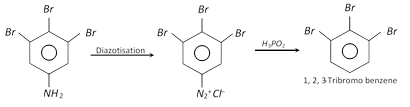
(ii) For the industrial preparation of important organic compounds like m-bromotoluene, m-bromophenol, etc.
(iii) For the preparation of a variety of useful halogen substituted arenes.
You need to login to perform this action.
You will be redirected in
3 sec
