General Methods and Mechanism of Polymerisation
Category : JEE Main & Advanced
(1) Chain growth or addition polymerisation : It involve a series of reaction each of which consumes a reactive particle and produces another similar one. The reactive particle may be free radicals or ion (cation or anion) to which monomers get added by a chain reaction. The polymers thus formed are known as chain growth polymers. Chain growth polymerisation is an important reaction of alkenes and conjugated dienes or indeed of all kinds of compounds that contain carbon-carbon double bond polythene, polypropylene, polybutadiene, teflon PVC, polystyrene are some of chain growth polymers. It is based on three mechanism
(i) Free radical mechanism
(ii) Cation mechanism
(iii) Anion mechanism
Each mechanism of polymerisation reaction involves an initiator of their corresponding nature. The addition polymerisation reaction is very rapid and is also characterized by three steps i.e. chain initiation, chain propogation and chain termination step.
(i) Free-radical mechanism : Free-radical polymerisation is initiated by organic peroxide or other reagents which decompose to give free radicals. Following steps are involved.
(a) Chain initiation : Organic peroxides undergo homolytic fission to form free radicals.
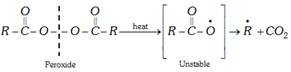
(b) Chain propagation : Free radical adds to an alkene molecule to form a new free radical.
![]()
The free radical formed attacks another alkene molecule and the process continues in building a long chain.
![]()
(c) Chain termination : The chain reaction comes to halt when two free radical chains combine.
\[2R{{(C{{H}_{2}}C{{H}_{2}})}_{n}}C{{H}_{2}}\overset{\bullet }{\mathop{C}}\,{{H}_{2}}\to R{{(C{{H}_{2}}C{{H}_{2}})}_{n}}C{{H}_{2}}C{{H}_{2}}:C{{H}_{2}}C{{H}_{2}}{{(C{{H}_{2}}C{{H}_{2}})}_{n}}R\]\[:C{{H}_{2}}C{{H}_{2}}{{(C{{H}_{2}}C{{H}_{2}})}_{n}}R\]
Free radical polymerisation can also be initiated by a mixture of ferrous sulphate and hydrogen peroxide \[(FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}})\].
(ii) Cationic mechanism : Cationic polymerisation is initiated by use of acids such as \[{{H}_{2}}S{{O}_{4}}\], HF or \[B{{F}_{3}}\] in \[{{H}_{2}}O\]. The following steps are involved :
(a) Chain initiation : The acid furnishes proton.
\[{{H}_{2}}S{{O}_{4}}\rightleftharpoons {{H}^{+}}+HSO_{4}^{-}\] \[HF\rightleftharpoons {{H}^{+}}+{{F}^{-}}\] \[B{{F}_{3}}+{{H}_{2}}O\rightleftharpoons {{H}^{+}}+B{{F}_{3}}{{(OH)}^{-}}\]
The proton adds to the carbon of the double bond of the alkene to form a carbonium ion.
(b) Chain propagation : The carbonium ion combines with another molecule of alkene to form a new carbonium ion and the process continues to form a long chain.
![]()
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}+nC{{H}_{2}}=C{{H}_{2}}\to C{{H}_{3}}C{{H}_{2}}{{(C{{H}_{2}}C{{H}_{2}})}_{n}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]= CH2\[\to C{{H}_{3}}C{{H}_{2}}{{(C{{H}_{2}}C{{H}_{2}})}_{n}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
(c) Chain termination : The chain may be halted by combination with negative ion or loss of a proton.
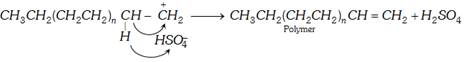
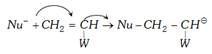
(iii) Anionic polymerisation : This type of polymerisation is initiated by anion (Bases nucleophiles) it proceeds through the formation of carbanion. The initiation may be brought about by \[{{K}^{+}}\bar{N}{{H}_{2}}\] of \[{{L}^{+}}N{{\bar{H}}_{2}}\].
The following steps are involved
(a) Chain initiation :
(b) Chain propagation :
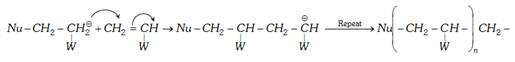
(c) Termination :
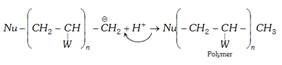
Chain transfer agents : In Vinylic polymerisation various other molecules react with main growing chain to interrupt the further growth of the original chain. This leads to lowering of average molecular mass of the polymer such reagents are called chain transfer agents. The common example \[CC{{l}_{4}},CB{{r}_{4}}\] etc.
For example in the presence of \[CC{{l}_{4}}\] styrene polymerizes to form a polymer of lower average molecular mass which also contains some chlorine.
\[C{{H}_{2}}=\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,H\,\,\,}}\,\xrightarrow{\text{Initiator}}\overset{\bullet }{\mathop{C}}\,{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{\overset{\bullet }{\mathop{C}}\,}}\,H\,\,\,}}\,\to \overset{\bullet }{\mathop{C}}\,{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,H-}}\,Cl+\overset{\bullet }{\mathop{C}}\,C{{l}_{3}}\]
\[C{{H}_{2}}=\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,H\,\,}}\,\xrightarrow{\overset{\bullet }{\mathop{C}}\,C{{l}_{3}}}C{{l}_{3}}C-C{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\overset{\bullet }{\mathop{C}}}\,H}}\,\xrightarrow{\text{Styrene}}\,{{\left( C{{l}_{3}}C-C{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}\,\,}{\mathop{\underset{|}{\mathop{C}}\,H-}}\,C{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-\,\,\,}}\, \right)}_{n}}\]\[\xrightarrow{\text{Styrene}}\,{{\left( C{{l}_{3}}C-C{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}\,\,}{\mathop{\underset{|}{\mathop{C}}\,H-}}\,C{{H}_{2}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-\,\,\,}}\, \right)}_{n}}\]
Chain transfer agents determinate chain reaction and inhibit further polymerisation and are also called inhibitors.
(2) Step growth or condensation polymerisation : In this type of polymerisation monomers generally contain two functional groups, i.e., difunctional monomers. In this process no initiator is needed and each step is the same type of chemical reaction. Since in this polymerisation reaction the polymer is formed in a stepwise manner. It is called step growth polymer and the process is called step growth polymerisation. The process for two monomer A and B may be expressed as.
\[\underset{\text{Monomers}}{\mathop{A+B}}\,\xrightarrow{\text{Condense}}\underset{\text{Dimer}}{\mathop{A-B}}\,\]
\[A-B+A\xrightarrow{\text{Condense}}\underset{\text{Trimer}}{\mathop{A-B-A}}\,\]
\[A-B-A+B\xrightarrow{{}}A-B-A-B\]
Alternatively, step growth can proceed as
\[A+B\to A-B\]
\[A-B+A-B\to A-B-A-B\text{ or }{{(A-B)}_{2}}\]
\[A-B-A-B+A-B-A-B\xrightarrow[\text{Polymer}]{}{{(A-B)}_{n}}\]
Some common examples of step growth polymers are
| Polymers | Monomers |
| Nylon-66 | Hexamethylenediamine and adipic acid |
| Bakelite | Phenol and formaldehyde |
| Dacron (polyester) | Terephthalic acid and ethylene glycol |
You need to login to perform this action.
You will be redirected in
3 sec
