Chromium containing compounds
Category : JEE Main & Advanced
Potassium dichromate, \[({{K}_{2}}C{{r}_{2}}{{O}_{7}})\]
Potassium dichromate is one of the most important compound of chromium, and also among dichromates. In this compound Cr is in the hexavalent (+6) state.
Preparation : It can be prepared by any of the following methods,
(i) From potassium chromate : Potassium dichromate can be obtained by adding a calculated amount of sulphuric acid to a saturated solution of potassium chromate.
\[\underset{\underset{\left( yellow \right)}{\mathop{potassium\,chromate}}\,}{\mathop{2{{K}_{2}}Cr{{O}_{4}}}}\,+{{H}_{2}}S{{O}_{4}}\to \underset{\underset{\left( orange \right)}{\mathop{potassium\,dichromate}}\,}{\mathop{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}\,+{{K}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] Crystals can be obtained by concentrating the solution and crystallisation.
(ii) Manufacture from chromite ore : \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] is generally manufactured from chromite ore \[(FeC{{r}_{2}}{{O}_{4}})\]. The process involves the following steps.
(a) Preparation of sodium chromate : Finely powdered chromite ore is mixed with soda ash and quicklime. The mixture is then roasted in a reverberatory furnace in the presence of air. Yellow mass due to the formation of sodium chromate is obtained.
\[4FeC{{r}_{2}}{{O}_{4}}+{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}+4C{{r}_{2}}{{O}_{3}}\]
\[\frac{\frac{4C{{r}_{2}}{{O}_{3}}+8N{{a}_{2}}C{{O}_{3}}+6{{O}_{2}}\to 8N{{a}_{2}}Cr{{O}_{4}}+8C{{O}_{2}}(g)}{4FeC{{r}_{2}}{{O}_{4}}+8N{{a}_{2}}C{{O}_{3}}+7{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}+8C{{O}_{2}}\left( g \right)+\underset{sodium\,chromate}{\mathop{8N{{a}_{2}}Cr{{O}_{4}}}}\,}}{{}}\]
The yellow mass is extracted with water, and filtered. The filtrate contains sodium chromate.
The reaction may also be carried out by using NaOH instead of \[N{{a}_{2}}C{{O}_{3}}\]. The reaction in that case is,
\[4FeC{{r}_{2}}{{O}_{4}}+16NaOH+7{{O}_{2}}\to 8N{{a}_{2}}Cr{{O}_{4}}+2F{{e}_{2}}{{O}_{3}}+8{{H}_{2}}O\]
(b) Conversion of chromate into dichromate : Sodium chromate solution obtained in step (a) is treated with concentrated sulphuric acid when it is converted into sodium dichromate.
\[\underset{sodium\,chromate}{\mathop{2N{{a}_{2}}Cr{{O}_{4}}}}\,+{{H}_{2}}S{{O}_{4}}\to \underset{sodium\,dichromate}{\mathop{N{{a}_{2}}C{{r}_{2}}{{O}_{7}}}}\,+N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
On concentration, the less soluble sodium sulphate, \[N{{a}_{2}}S{{O}_{4}}.10{{H}_{2}}O\] crystallizes out. This is filtered hot and allowed to cool when sodium dichromate, \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}.2{{H}_{2}}O,\] separates out on standing.
(c) Concentration of sodium dichromate to potassium dichromate : Hot concentrated solution of sodium dichromate is treated with a calculated amount of potassium chloride. When potassium dichromate being less soluble crystallizes out on cooling.
\[\underset{sod.dichromate}{\mathop{N{{a}_{2}}C{{r}_{2}}{{O}_{7}}+}}\,2KCl\to \underset{pot.dichromate}{\mathop{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}\,+2NaCl\]
Physical properties
(i) Potassium dichromate forms orange-red coloured crystals.
(ii) It melts at 699 K.
(iii) It is very stable in air (near room temperature) and is generally, used as a primary standard in the volumetric analysis.
(iv) It is soluble in water though the solubility is limited.
Chemical properties
(i) Action of heat : Potassium dichromate when heated strongly. Decomposes to give oxygen.
\[4{{K}_{2}}C{{r}_{2}}{{O}_{7}}\left( s \right)\xrightarrow{\Delta }4{{K}_{2}}Cr{{O}_{4}}(s)+2C{{r}_{2}}{{O}_{3}}(s)+3{{O}_{2}}\]
(ii) Action of acids
(a) In cold, with concentrated H2SO4, red crystals of chromium trioxide separate out.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}(aq)+conc.{{H}_{2}}S{{O}_{4}}\to KHS{{O}_{4}}(aq)+2Cr{{O}_{3}}\left( s \right)+{{H}_{2}}O\]
On heating a dichromate-sulphuric acid mixture, oxygen gas is given out.
\[2{{K}_{2}}C{{r}_{2}}{{O}_{7}}+8{{H}_{2}}S{{O}_{4}}\to 2{{K}_{2}}S{{O}_{4}}+2C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+8{{H}_{2}}O+3{{O}_{2}}\]
(b) With HCl, on heating chromic chloride is formed and \[C{{l}_{2}}\] is liberated.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7\left( aq \right)}}+14HCl\left( aq \right)\to 2CrC{{l}_{3\left( aq \right)}}+2KCl\left( aq \right)+7{{H}_{2}}O+3C{{l}_{2}}\left( g \right)\]
(iii) Action of alkalies : With alkalies, it gives chromates. For example, with KOH,
\[\underset{orange}{\mathop{{{K}_{2}}C{{r}_{2}}{{O}_{4}}}}\,+2KOH\to \underset{yellow}{\mathop{2{{K}_{2}}Cr{{O}_{4}}}}\,+{{H}_{2}}O\]
On acidifying, the colour again changes to orange-red owing to the formation of dichromate.
\[2{{K}_{2}}Cr{{O}_{4}}+{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{K}_{2}}S{{O}_{4}}+{{H}_{2}}O\]
Actually, in dichromate solution, the \[C{{r}_{2}}O_{7}^{2-}\]ions are in equilibrium with \[CrO_{4}^{2-}\]ions.
\[C{{r}_{2}}O_{7}^{2-}+{{H}_{2}}O\]\[\rightleftharpoons \]\[2CrO_{4}^{2-}+2{{H}^{+}}\]
(iv) Oxidising nature : In neutral or in acidic solution, potassium dichromate acts as an excellent oxidising agent, and \[C{{r}_{2}}O_{7}^{2-}\]gets reduced to \[C{{r}^{3+}}\]. The standard electrode potential for the reaction,
\[C{{r}_{2}}O_{7}^{2-}+14{{H}^{+}}+6{{e}^{-}}\to 2C{{r}^{+3}}+7{{H}_{2}}O\] is +1.31V.
This indicates that dichromate ion is a fairly strong oxidising agent, especially in strongly acidic solutions. That is why potassium dichromate is widely used as an oxidising agent, for quantitative estimation of the reducing agents such as, Fe2+. It oxidises,
(a) Ferrous salts to ferric salts
\[{{K}_{2}}Cr{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+4{{H}_{2}}O+3\left[ O \right]\]
\[\frac{2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+\left[ O \right]\to F{{e}_{2}}{{\left[ S{{O}_{4}} \right]}_{3}}+{{H}_{2}}O\times 3}{{{K}_{2}}C{{r}_{2}}{{O}_{7}}+6FeS{{O}_{4}}+7{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+3F{{e}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+7{{H}_{2}}O}\]
Ionic equation
\[C{{r}_{2}}O_{7}^{2-}+14{{H}^{+}}+6F{{e}^{2+}}\to 2C{{r}^{3+}}+6F{{e}^{3+}}+7{{H}_{2}}O\]
(b) Sulphites to sulphates and arsenites to arsenates.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+4{{H}_{2}}O+3\left[ O \right]\]
\[\frac{\frac{N{{a}_{2}}S{{O}_{3}}+[O]\to N{{a}_{2}}S{{O}_{4}}]\times 3}{{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}+3N{{a}_{2}}S{{O}_{3}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+3N{{a}_{2}}S{{O}_{4}}+4{{H}_{2}}O}}{{}}\]
Ionic equation
\[C{{r}_{2}}O_{7}^{2-}+8{{H}^{+}}+3SO_{3}^{2-}\to 2C{{r}^{3+}}+3SO_{4}^{2-}+4{{H}_{2}}O\]
Similarly, arsenites are oxidised to arsenates.
\[C{{r}_{2}}O_{7}^{2-}+8{{H}^{+}}+3AsO_{3}^{3-}\to 2C{{r}^{3+}}+3AsO_{4}^{3-}+4{{H}_{2}}O\]
(c) Hydrogen halides to halogens.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+4{{H}_{2}}O+3\left[ O \right]\]
\[\frac{\frac{2HX+O\to {{H}_{2}}O+{{X}_{2}}]\times 3}{{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}+6HX\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+7{{H}_{2}}O+3{{X}_{2}}}}{{}}\]
where, X may be Cl, Br, I.
Ionic equation : \[C{{r}_{2}}O_{7}^{2-}+8{{H}^{+}}+6HX\to 2C{{r}^{3+}}+3{{X}_{2}}+7{{H}_{2}}O\]
(d) Iodides to iodine
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+4{{H}_{2}}O+3\left[ O \right]\]
\[2KI+{{H}_{2}}O+\left[ O \right]\to 2KOH+{{I}_{2}}]\times \]3
\[\frac{2KOH+{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+2{{H}_{2}}O]\,\,\times \,\,3}{{{K}_{2}}C{{r}_{2}}{{O}_{7}}+7{{H}_{2}}S{{O}_{4}}+6KI\to 4{{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+3{{I}_{2}}+7{{H}_{2}}O}\]
Ionic equation : \[C{{r}_{2}}O_{7}^{2-}+14{{H}^{+}}+6{{I}^{-}}\to 2C{{r}^{3+}}+7{{H}_{2}}O+3{{I}_{2}}\]
Thus, when KI is added to an acidified solution of K2Cr2O7 iodine gets liberated.
(e) It oxidises H2S to S.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+4{{H}_{2}}O+3\left[ O \right]\]
\[{{H}_{2}}S+\left[ O \right]\to {{H}_{2}}O+S]\times 3\] \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}+3{{H}_{2}}S\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}+7{{H}_{2}}O+3S\]
Ionic equation
\[C{{r}_{2}}O_{7}^{2-}+8{{H}^{+}}+3{{H}_{2}}S\to 2C{{r}^{3+}}+3S+7{{H}_{2}}O\]
(v) Formation of insoluble chromates : With soluble salts of lead, barium etc., potassium dichromate gives insoluble chromates. Lead chromate is an important yellow pigment.
\[2Pb{{\left( N{{O}_{3}} \right)}_{2}}+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{H}_{2}}O\to 2PbCr{{O}_{4}}+2KN{{O}_{3}}+2HN{{O}_{3}}\]
(vi) Chromyl chloride test : When potassium dichromate is heated with conc. \[{{H}_{2}}S{{O}_{4}}\] in the presence of a soluble chloride salt, the orange-red vapours of chromyl chloride \[(Cr{{O}_{2}}C{{l}_{2}})\] are formed.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4NaCl+6{{H}_{2}}S{{O}_{4}}\xrightarrow{heat}\]\[2KHS{{O}_{4}}+4NaHS{{O}_{4}}+\underset{\underset{\left( orange-red\,vapours\, \right)}{\mathop{chromyl\,chloride}}\,}{\mathop{2Cr{{O}_{2}}C{{l}_{2}}}}\,\]
Chromyl chloride vapours when passed through water give yellow-coloured solution containing chromic acid.
\[Cr{{O}_{2}}C{{l}_{2}}+2{{H}_{2}}O\to 2HCl+\underset{Chromic\,acid.\,(yellow\,solution)\,}{\mathop{{{H}_{2}}Cr{{O}_{4}}}}\,\]
Chromyl chloride test can be used for the detection of chloride ion is any mixture.
Uses : Potassium dichromate is used as,
(i) An oxidising agent
(ii) In chrome tanning
(iii) The raw meterial for preparing large number of chromium compounds
(iv) Primary standard in the volumetric analysis.
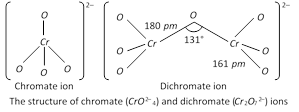
Structures of Chromate and Dichromate Ions
Chromates and dichromates are the salts of chromic acid \[({{H}_{2}}Cr{{O}_{4}})\]. In solution, these ions exist in equilibrium with each other. Chromate ion has four oxygen atoms arranged tetrahedrally around Cr atom. (see Fig). Dichromate ion involves a \[Cr-O-Cr\] bond as shown in Fig.
You need to login to perform this action.
You will be redirected in
3 sec
