Electro Chemical Cell
Category : JEE Main & Advanced
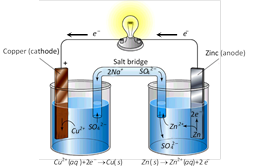
It is an arrangement in which the chemical energy is converted into electrical energy due to chemical action taking place in it.
(1) Primary cell : Is that cell in which electrical energy is produced due to chemical energy. In the primary cell, chemical reaction is irreversible. This cell can not be recharged. Examples of primary cells are Voltaic cell, Daniel cell, Leclanche cell and Dry cell etc.
(2) Secondary cell : A secondary cell is that cell in which the electrical energy is first stored up as a chemical energy and when the current is taken from the cell, the chemical energy is reconverted into electrical energy. In the secondary cell chemical reactions are reversible. The secondary cells are also called storage cell or accumulator. The commonly used secondary cells is lead accumulator.
(3) Defects In a primary cell : In voltaic cell there are two main defects arises.
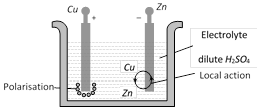
Local action : It arises due to the presence of impurities of iron, carbon etc. on the surface of commercial Zn rod used as an electrode. The particles of these impurities and Zn in contact with sulphuric acid form minute voltaic cell in which small local electric currents are set up resulting in the wastage of Zn even when the cell is not sending the external current.
Removal : By amalgamating Zn rod with mercury (i.e. the surface of Zn is coated with Hg).
Polarisation : It arises, when the positive \[{{H}_{2}}\] ions, which are formed by the action of Zn on sulphuric acid, travel towards the Cu rod and after transferring, the positive charge converted into H2 gas atoms and get deposited in the form of neutral layer of a gas on the surface of Cu rod. This weakens the action of cell.
Removal : Either by brushing the anode the remove the layer or by using a depolariser (i.e. some oxidising agent \[Mn{{O}_{2}},\,CuS{{O}_{4}}\] etc which may oxidise \[{{H}_{2}}\] into water).
Thermo electric effect of current

If two wires of different metals are joined at their ends so as to form two junctions, then the resulting arrangement is called a "Thermo couple".
You need to login to perform this action.
You will be redirected in
3 sec
