Maxwellís Law (or the Distribution of Molecular Speeds
Category : JEE Main & Advanced
(1) The \[{{v}_{rms}}\] gives us a general idea of molecular speeds in a gas at a given temperature. This doesn't mean that the speed of each molecule is \[{{v}_{rms}}\]. Many of the molecules have speed less than \[{{v}_{rms}}\] and many have speeds greater than \[{{v}_{rms}}\].
(2) Maxwell derived as equation given the distribution of molecules in different speed as follow
\[dN=4\pi N\,{{\left( \frac{m}{2\pi kT} \right)}^{3/2}}{{v}^{2}}{{e}^{-\frac{m{{v}^{2}}}{2kT}}}dv\]
where dN = Number of molecules with speeds between v and v + dv.
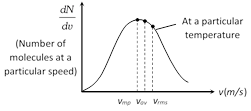
(3) Graph between \[\frac{dN}{dv}\] (number of molecules at a particular speed) and v (speed of these molecules). From the graph it is seen that \[\frac{dN}{dv}\] is maximum at most probable speed.
This graph also represent that \[{{v}_{rms}}>{{v}_{av}}>{{v}_{mp}}\]
(Order remember trick RAM)
\[\Rightarrow \]\[\sqrt{\frac{3RT}{M}}\,>\sqrt{\frac{8RT}{\pi M}}\,>\,\sqrt{\frac{2RT}{M}}\]\[\Rightarrow \]\[1.77\sqrt{\frac{RT}{M}}>1.6\sqrt{\frac{RT}{M}}\,>\,1.41\sqrt{\frac{RT}{M}}\]
Area bonded by this curve with speed axis represents the number of molecules corresponds to that velocity range. This curve is asymmetric curve.
Effect of temperature on velocity distribution : With temperature rise the \[\frac{dN}{dv}\,\text{vs}\,v\]. Curve shift towards right and becomes broader.
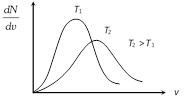
(Because with temperature rise average molecular speed increases).
You need to login to perform this action.
You will be redirected in
3 sec
