Thomson's Mass Spectrograph
Category : JEE Main & Advanced
It is used to measure atomic masses of various isotopes in gas. This is done by measuring q/m of singly ionised positive ion of the gas.
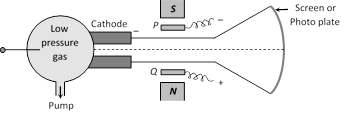
(1) The positive ions are produced in the bulb at the left hand side. These ions are accelerated towards cathode. Some of the positive ions pass through the fine hole in the cathode. This fine ray of positive ions is subjected to electric field E and magnetic field B and then allowed to strike a fluorescent screen (\[\vec{E}||\vec{B}\] but \[\vec{E}\]or \[\vec{B}\ \bot \ \vec{v}\]).
(2) If the initial motion of the ions is in \[+x\] direction and electric and magnetic fields are applied along \[+y\] axis then force due to electric field is in the direction of y-axis and due to magnetic field it is along z-direction.
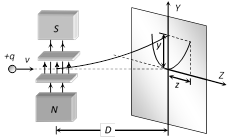
The deflection due to electric field alone \[y=\frac{qELD}{m{{v}^{2}}}\] .....(i)
The deflection due to magnetic field alone \[z=\frac{qBLD}{mv}\] .....(ii)
From equation (i) and (ii), \[{{z}^{2}}=k\left( \frac{q}{m} \right)y\]
where \[k=\frac{{{B}^{2}}LD}{E}\]; This is the equation of parabola. It means all the charged particles moving with different velocities but of same q/m value will strike the screen placed in yz plane on a parabolic track as shown in the above figure.
(3) All the positive ions of same. q/m moving with different velocity lie on the same parabola. Higher is the velocity lower is the value of y and z. The ions of different specific charge will lie on different parabola.
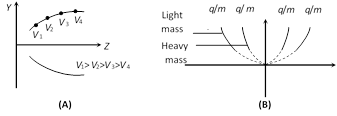
(4) The number of parabola tells the number of isotopes present in the given ionic beam.
You need to login to perform this action.
You will be redirected in
3 sec
