Conduction in Metallic Rod
Category : JEE Main & Advanced
When one end of a metallic rod is heated, heat flows by conduction from the hot end to the cold end.
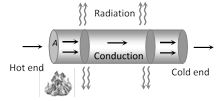
(1) Variable state : In this state Temperature of every part of the rod increases.
Heat received by each cross-section of the rod from hotter end used in three ways.
(i) A part increases temperature of itself.
(ii) Another part transferred to neighbouring cross-section.
(iii) Remaining part radiates.
(2) Steady state : After sometime, a state is reached when the temperature of every cross-section of the rod becomes constant. In this state, no heat is absorbed by the rod. The heat that reaches any cross-section is transmitted to the next except that a small part of heat is lost to surrounding from the sides by convection & radiation. This state of the rod is called steady state.
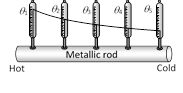
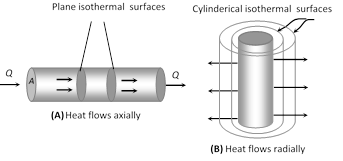
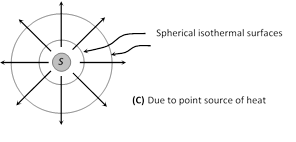
(3) Isothermal surface : Any surface (within a conductor) having its all points at the same temperature, is called isothermal surface. The direction of flow of heat through a conductor at any point is perpendicular to the isothermal surface passing through that point.
(4) Temperature gradient (T.G.) : The rate of change of temperature with distance between two isothermal surfaces is called temperature gradient. Hence
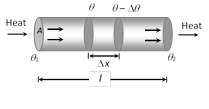
(i) Temperature gradient \[=\frac{-\Delta \theta }{\Delta x}\]
(ii) The negative sign show that temperature \[\theta \] decreases as the distance x increases in the direction of heat flow.
(iii) For uniform temperature fall \[\frac{{{\theta }_{1}}-{{\theta }_{2}}}{l}=\frac{\Delta \theta }{\Delta x}\]
(iv) Unit : K/m or \[^{o}C/m\] (S.I.) and Dimensions \[[{{L}^{-1}}\theta ]\]
(5) Law of thermal conductivity : Consider a rod of length l and area of cross-section A whose faces are maintained at temperature \[{{\theta }_{1}}\] and \[{{\theta }_{2}}\] respectively. The curved surface of rod is kept insulated from surrounding to avoid leakage of heat
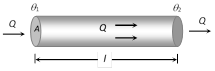
(i) In steady state the amount of heat flowing from one face to the other face in time t is given by \[Q=\frac{KA({{\theta }_{1}}-{{\theta }_{2}})\,t}{l}\]
where K is coefficient of thermal conductivity of material of rod.
(ii) Rate of flow of heat i.e. heat current \[\frac{Q}{t}=H\]\[=\frac{KA({{\theta }_{1}}-{{\theta }_{2}})\,}{l}\]
(iii) In case of non-steady state or variable cross-section, a more general equation can be used to solve problems.
\[\frac{dQ}{dt}=-\,KA\frac{d\theta }{dx}\]
(6) More about K : It is the measure of the ability of a substance to conduct heat through it.
(i) Units : Cal/cm-sec \[^{o}C\] (in C.G.S.), kcal/m-sec-K (in M.K.S.) and W/m- K (in S.I.). Dimension : \[[ML{{T}^{-3}}{{\theta }^{-1}}]\]
(ii) The magnitude of K depends only on nature of the material.
(iii) Substances in which heat flows quickly and easily are known as good conductor of heat. They possesses large thermal conductivity due to large number of free electrons e.g. Silver, brass etc. For perfect conductors, \[K=\infty \].
(iv) Substances which do not permit easy flow of heat are called bad conductors. They possess low thermal conductivity due to very few free electrons e.g. Glass, wood etc. and for perfect insulators, \[K=0\].
(v) The thermal conductivity of pure metals decreases with rise in temperature but for alloys thermal conductivity increases with increase of temperature.
(vi) Human body is a bad conductor of heat (but it is a good conductor of electricity).
(vii) Decreasing order of conductivity : For some special cases it is as follows
(a) \[{{K}_{Ag}}>{{K}_{Cu}}>{{K}_{Al}}\]
(b) \[{{K}_{Solid}}>{{K}_{Liquid}}>{{K}_{Gas}}\]
(c) \[{{K}_{Metals}}>{{K}_{Non-metals}}\]
Thermal conductivity of some material
| Substance | Thermal conductivity (W/m-K) | Substance | Thermal conductivity (W/m-K) |
| Aluminium | 240 | Concrete | 0.9 |
| Copper | 400 | Water | 0.6 |
| Gold | 300 | Glass wool | 0.04 |
| Iron | 80 | Air | 0.024 |
| Lead | 35 | Helium | 0.14 |
| Glass | 0.9 | Hydrogen | 0.17 |
| Wood | 0.1-0.2 | Oxygen | 0.024 |
(7) Relation between temperature gradient and thermal conductivity : In steady state, rate of flow of heat \[\frac{dQ}{dt}=-KA\frac{d\theta }{dx}\]\[=-KA\times (T.G.)\Rightarrow \mathbf{(T}\mathbf{.G}\mathbf{.)}\propto \frac{\mathbf{1}}{\mathbf{K}}\] (\[\frac{dQ}{dt}\]= constant) Temperature difference between the hot end and the cold end in steady state is inversely proportional to K, i.e. in case of good conductors temperature of the cold end will be very near to hot end.
In ideal conductor where \[k=\infty ,\] temperature difference in steady state will be zero.
(8) Thermal resistance (R) : The thermal resistance of a body is a measure of its opposition to the flow of heat through it.
It is defined as the ratio of temperature difference to the heat current (= Rate of flow of heat)
(i) Hence \[R=\frac{{{\theta }_{1}}-{{\theta }_{2}}}{H}=\frac{{{\theta }_{1}}-{{\theta }_{2}}}{KA({{\theta }_{1}}-{{\theta }_{2}})/l}\]\[=\frac{l}{KA}\]
(ii) Unit : \[^{o}C\times sec/cal\] or \[K\times sec/kcal\] and Dimension : \[[{{M}^{-1}}{{L}^{-2}}{{T}^{3}}\theta ]\]
(9) Wiedmann-Franz law : At a given temperature T, the ratio of thermal conductivity to electrical conductivity is constant i.e., \[(K/\sigma T)\]= constant, i.e., a substance which is a good conductor of heat (e.g., silver) is also a good conductor of electricity. Mica is an exception to above law.
(10) Thermometric conductivity or diffusivity : It is a measure of rate of change of temperature (with time) when the body is not in steady state (i.e., in variable state)
It is defined as the ratio of the coefficient of thermal conductivity to the thermal capacity per unit volume of the material. Thermal capacity per unit volume\[=\frac{mc}{V}=\rho \,c\]
(\[\rho \] = density of substance) \[\Rightarrow \] Diffusivity (D) = \[\frac{K}{\rho \,c}\]
Unit : \[{{m}^{2}}/\sec \] and Dimension : \[[{{L}^{2}}{{T}^{-1}}]\]
Electrical Analogy for Thermal Conduction
| Electrical conduction | Thermal conduction |
| Electric charge flows from higher potential to lower potential | Heat flows from higher temperature to lower temperature |
|
The rate of flow of charge is called the electric current, i.e. \[I=\frac{dq}{dt}\] |
The rate of flow of heat may be called as heat current i.e. \[H=\frac{dQ}{dt}\] |
|
The relation between the electric current and the potential difference is given by Ohm's law, that is \[I=\frac{{{V}_{1}}-{{V}_{2}}}{R}\] where R is the electrical resistance of the conductor |
Similarly, the heat current may be related with the temperature difference as \[H=\frac{{{\theta }_{1}}-{{\theta }_{2}}}{R}\] where R is the thermal resistance of the conductor |
|
The electrical resistance is defined as \[R=\frac{\rho l}{A}=\frac{l}{\sigma A}\] where \[\rho =\] Resistivity and \[\sigma =\] Electrical conductivity \[\frac{dq}{dt}=I=\frac{{{V}_{1}}-{{V}_{2}}}{R}=\frac{\sigma \,A}{l}({{V}_{1}}-{{V}_{2}})\] |
The thermal resistance may be defined as \[R=\frac{l}{KA}\] where K = Thermal conductivity of conductor \[\frac{dQ}{dt}=H=\frac{{{\theta }_{1}}-{{\theta }_{2}}}{R}=\frac{KA}{l}({{\theta }_{1}}-{{\theta }_{2}})\] |
You need to login to perform this action.
You will be redirected in
3 sec
