Trihydric Alcohols.
Category : JEE Main & Advanced
The only important trihydric alcohol is glycerol (propane-1, 2, 3-triol). It occurs as glycosides in almost all animal and vegetable oils and fats.
(1) Preparation
(i) From oils and fats
\[\underset{\text{Oil or fat}}{\mathop{\underset{C{{H}_{2}}OOCR}{\overset{C{{H}_{2}}OOCR}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,}{\mathop{CH}}}\,OOCR\,}}}\,}}\,+\underset{\text{steam}}{\mathop{3{{H}_{2}}O}}\,\to \underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,}}\,\,+\underset{\text{Fatty acids}}{\mathop{3RCOOH}}\,\]
\[\underset{\text{Oil or fat}}{\mathop{\underset{C{{H}_{2}}OOCR}{\overset{C{{H}_{2}}OOCR}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,}{\mathop{CH}}}\,OOCR\,}}}\,}}\,+\underset{NaOH}{\overset{NaOH}{\mathop{\underset{{}}{\overset{{}}{\mathop{NaOH}}}\,}}}\,\xrightarrow{\text{Hydrolysis}}\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,\]\[+\underset{\text{Sodium salt of higher fatty acids}}{\mathop{3RCOONa}}\,\]
(ii) By fermentation of sugar
\[\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{C}_{6}}}}\,\underset{N{{a}_{2}}S{{O}_{3}}}{\mathop{\xrightarrow{\text{Yeast}}}}\,\underset{\text{Glycerol}}{\mathop{{{C}_{3}}{{H}_{8}}{{O}_{3}}}}\,+\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\,\,+C{{O}_{2}}\]
(iii) From propene [Modern method]
\[\underset{\text{propene}}{\mathop{\underset{C{{H}_{2}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|\,|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,\,\,}}}\,}}\,\underset{{{600}^{o}}C}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{\text{Allyl chloride}}{\mathop{\underset{C{{H}_{2}}\,\,\,\,}{\overset{C{{H}_{2}}Cl}{\mathop{\underset{|\,|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{NaOH(\text{dil})}\underset{\text{Allyl alcohol}}{\mathop{\underset{C{{H}_{2}}\,\,\,\,\,\,}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
\[\xrightarrow{HOCl}\underset{\beta \text{-monochlorohydrin}}{\mathop{\underset{C{{H}_{2}}-OH}{\overset{C{{H}_{2}}OH\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,Cl\,\,\,\,\,}}}\,}}\,\xrightarrow{\text{aq}\text{. }NaOH}\underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}-OH}{\overset{C{{H}_{2}}-OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,-OH\,}}}\,}}\,\]
(iv) From propenal :
\[C{{H}_{2}}=CHCHO\underset{\text{catalyst}}{\mathop{\xrightarrow{{{H}_{2}}}}}\,C{{H}_{2}}=CHC{{H}_{2}}OH\] \[\xrightarrow{{{H}_{2}}{{O}_{2}}/\overset{}{\mathop{O}}\,H}\underset{\text{Glycerol}}{\mathop{HOC{{H}_{2}}CHOHC{{H}_{2}}OH}}\,\]
(2) Physical properties
(i) It is a colourless, odourless, viscous and hygroscopic liquid.
(ii) It has high boiling point i.e., \[{{290}^{o}}C\]. The high viscosity and high boiling point of glycerol are due to association through hydrogen bonding.
(iii) It is soluble in water and ethyl alcohol but insoluble in ether.
(iv) It is sweet in taste and non toxic in nature.
(3) Chemical properties
(i) Reaction with sodium
\[\underset{C{{H}_{2}}-OH}{\overset{C{{H}_{2}}-OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,-OH\,}}}\,\underset{\begin{smallmatrix}\text{Room}\\\text{temperature}\end{smallmatrix}}{\mathop{\xrightarrow{\,\,\,Na\,\,\,}}}\,\underset{\text{Monosodium glycerol}}{\mathop{\underset{C{{H}_{2}}-OH}{\overset{C{{H}_{2}}ONa\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,-OH\,}}}\,}}\,\underset{\begin{smallmatrix}\text{Room}\\\text{temperature}\end{smallmatrix}}{\mathop{\xrightarrow{\,\,\,Na\,\,\,}}}\,\underset{\text{Disodiumglycerolate}}{\mathop{\underset{C{{H}_{2}}ONa}{\overset{C{{H}_{2}}ONa}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,-OH}}}\,}}\,\]
(ii) Reaction with \[\mathbf{PC}{{\mathbf{l}}_{\mathbf{5}}}\mathbf{,}\]\[\mathbf{PB}{{\mathbf{r}}_{\mathbf{3}}}\] and \[\mathbf{P}{{\mathbf{l}}_{\mathbf{3}}}\]
(a) \[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,\,\,+\,\,\,\,3PC{{l}_{5}}\underset{\begin{smallmatrix}\text{Glyceryl trichloride} \\\text{(1, 2, 3-Trichloropropane)}\end{smallmatrix}}{\mathop{\to\,\,\,\,\,\,\underset{C{{H}_{2}}Cl}{\overset{C{{H}_{2}}Cl}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,Cl}}}\,}}\,+\,\,\,\,3POC{{l}_{3}}+\,\,\,3HCl\]
(b) \[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,\,\,+\,\,\,\,PB{{r}_{3}}\underset{\text{1, 2, 3-Tribromopropane}}{\mathop{\to\,\,\,\,\,\,\underset{C{{H}_{2}}Br}{\overset{C{{H}_{2}}Br}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,Br}}}\,}}\,+\,\,\,\,{{H}_{3}}P{{O}_{3}}\]
(c) \[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,\,\,+\,\,\,\,P{{I}_{3}}\to \underset{\text{(Unstable)}}{\mathop{\left[\underset{C{{H}_{2}}I}{\overset{C{{H}_{2}}I}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,I}}}\,\right]}}\,\to\underset{\text{Allyliodide}}{\mathop{\underset{C{{H}_{2}}I}{\overset{C{{H}_{2}}}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,|\,\,\,\,}{\mathop{CH}}}\,\,\,\,}}}\,}}\,+\,\,\,{{I}_{2}}\]
(iii) Reaction with HCl or HBr
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\underset{+HCl}{\mathop{\xrightarrow{{{110}^{o}}C}}}\,\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}Cl\,\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,+\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,Cl\,}}}\, \\ & \alpha \text{-Glycerol monochlorohydrin (66 }\!\!%\!\!\text{ )}\beta \text{-Glycerol monochlorohydrin (34 }\!\!%\!\!\text{ )} \\ \end{align}\] \[\begin{align}
& \underset{{{110}^{o}}C}{\mathop{\xrightarrow{\text{Excess of }HCl}}}\,\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}Cl\,\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,Cl\,\,\,\,}}}\,+\underset{C{{H}_{2}}Cl}{\overset{C{{H}_{2}}Cl\,\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH}}}\, \\ & \,\,\text{Glycerol }\alpha ,\,\beta \text{-dichlorohydrin (56 }\!\!%\!\!\text{ )}\text{Glycerol }\alpha ,\,{\alpha }'\text{-dichlorohydrin v(44 }\!\!%\!\!\text{ )} \\ \]
(iv) Reaction with HI
(a) \[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,+3HI\underset{\begin{smallmatrix}\text{ 1,2,3-Tri-iodopropane} \\\text{ (Unstable)}\end{smallmatrix}}{\mathop{\,\,\,\xrightarrow{\text{Warm}}\,\,\,\,\underset{C{{H}_{2}}I}{\overset{C{{H}_{2}}I}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,I\,}}}\,\,\,\,\,\,}}\,\,\to \,\,\,\,\,\underset{\text{Allyl iodide}}{\mathop{\underset{C{{H}_{2}}I}{\overset{C{{H}_{2}}\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,|\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,\,}}}\,}}\,+\,\,\,\,\,{{I}_{2}}\]
(b) \[\underset{\text{Allyliodide}}{\mathop{\underset{C{{H}_{2}}I}{\overset{C{{H}_{2}}\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\overset{|\,|\,\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,\,}}}\,}}\,+\,\,HI\to\underset{\text{Unstable}}{\mathop{\underset{C{{H}_{2}}I}{\overset{C{{H}_{3}}\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,I\,\,\,\,}}}\,}}\,\xrightarrow{-{{I}_{2}}}\underset{\text{Propene}}{\mathop{\underset{C{{H}_{2}}\,\,}{\overset{C{{H}_{3}}\,\,}{\mathop{\underset{|\,|\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,\,}}}\,}}\,\xrightarrow{HI}\underset{\text{Isopropyliodide}}{\mathop{\,\,\,\,\,\,\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,I\,}}}\,\,\,\,\,\,\,\,}}\,\]
(v) Reaction with oxalic acid
(a) At \[{{110}^{o}}C\] Glycerol is formed
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,+\underset{\text{Oxalic acid}}{\mathop{HOOC-COOH}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{100-{{110}^{o}}C}}}\,\underset{\text{Glycerol mono-oxalate}}{\mathop{\underset{C{{H}_{2}}OH\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{C{{H}_{2}}OOC\,COOH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\] \[\xrightarrow{-C{{O}_{2}}}\underset{\text{Glycerol mono formate}}{\mathop{\underset{C{{H}_{2}}-OH\,\,\,\,\,}{\overset{C{{H}_{2}}O-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-H}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,-OH\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,}}\,+\underset{\text{Formic acid}}{\mathop{H\,COOH}}\,\]
(b) At \[{{260}^{o}}C,\] allyl alcohol is formed
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,+\begin{matrix}\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{HOOC}}\, \\HOOC \\\end{matrix}\xrightarrow{-2{{H}_{2}}O}\underset{C{{H}_{2}}OH\,\,\,}{\overset{C{{H}_{2}}OOC}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OO\overset{|}{\mathop{C}}\,\,}}}\,\] \[\xrightarrow{-2C{{O}_{2}}}\underset{\text{Allylalcohol}}{\mathop{\overset{C{{H}_{2}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,|\,\,\,\,\,}{\mathop{CH}}\,-C{{H}_{2}}OH}}\,}}\,\]
(vi) Dehydration
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,\,\underset{\Delta }{\mathop{\xrightarrow{\text{conc}\text{. }{{H}_{2}}S{{O}_{4}}/{{P}_{2}}{{O}_{5}}/KHS{{O}_{4}}}}}\,\underset{\begin{smallmatrix}\text{Acrolene or} \\ \text{allyl aldehyde}\end{smallmatrix}}{\mathop{\underset{CHO}{\overset{C{{H}_{2}}\,\,}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,|\,\,\,\,\,}{\mathop{CH}}}\,\,\,\,\,}}}\,}}\,+\,\,\,2{{H}_{2}}O\]
(vii) Oxidation
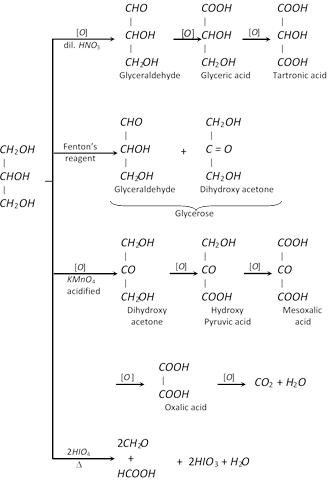
(viii) Reaction with nitric acid
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,+\,\,3HN{{O}_{3}}\xrightarrow{\text{conc}\text{. }{{H}_{2}}S{{O}_{4}}}\underset{\text{Glyceryl trinitrate (T}\text{.N}\text{.G}\text{.)}}{\mathop{\underset{C{{H}_{2}}ON{{O}_{2}}}{\overset{C{{H}_{2}}ON{{O}_{2}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,ON{{O}_{2}}}}}\,\,\,+\,\,3{{H}_{2}}O}}\,\]
Dynamite is prepared from T.N.G.
Dynamite : A mixture of T.N.G. and glyceryl dinitrate absorbed in kieselguhr is called dynamite. It was discovered by Alfred. Nobel in 1867. It releases large volume of gases and occupy 10,900 times the volume of nitroglycerine.
\[{{C}_{3}}{{H}_{5}}{{(ONO)}_{3}}\to 12C{{O}_{2}}+10{{H}_{2}}O+6{{N}_{2}}+{{O}_{2}}\]
Blasting gelatin : A mixture of glyceryl trinitrate and cellulose nitrate (gun cotton).
Cordite : It is obtained by mixing glyceryl trinitrate with gun cotton and vaseline it is smokeless explosive.
(4) Uses
(a) As antifreeze in automobile radiator.
(b) In the preparation of good quality of soap-hand lotions shaving creams and tooth pastes.
(c) As a lubricant in watches.
(d) As a preservatives.
(e) As a sweetening agent in confectionary, beverages and medicines being non toxic in nature.
(f) In manufacture of explosives such as dynamite.
(5) Analytical tests of glycerol
(i) Acrolein test : When glycerol is heated with \[KHS{{O}_{4}}\] a very offensive smell is produced due to formation of acrolein. Its aqueous solution restores the colour of schiff’s reagent and reduces Fehling solution and Tollen’s reagent.
(ii) Dunstan’s test : A drop of phenolphthalein is added approximately 5 ml of borax solution. The pink colour appears on adding 2-3 drops of glycerol, pink colour disappears. The pink colour appears on heating and disappears on cooling again.
You need to login to perform this action.
You will be redirected in
3 sec
