Preparation Of Carbonyl Compounds
Category : JEE Main & Advanced
(1) From alcohols
(i) By oxidation.
\[\underset{\text{Secondary alcohol}}{\mathop{\overset{OH}{\mathop{\overset{|\,\,\,\,\,\,}{\mathop{R-CH-R'}}\,}}\,}}\,\underset{\text{agents}}{\mathop{\xrightarrow{\text{Mild oxidising}}}}\,\underset{\text{Ketone}}{\mathop{\overset{O}{\mathop{\overset{||}{\mathop{R-C-R'}}\,}}\,}}\,\]
\[\underset{\text{Primary alcohol}}{\mathop{R-C{{H}_{2}}-OH}}\,\underset{\text{agents}}{\mathop{\xrightarrow{\text{Mild oxidising}}}}\,\underset{\text{Aldehyde}}{\mathop{\overset{O}{\mathop{\overset{||}{\mathop{R-C-H}}\,}}\,}}\,\]
Mild oxidising agents are
(a) \[{{X}_{2}}\] (Halogen)
(b) Fenton reagent (\[FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\])
(c) \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/\overset{\oplus }{\mathop{H}}\,\]
(d) Jones reagent
(e) Sarret reagent
(f) \[Mn{{O}_{2}}\]
(g) Aluminium tertiary butoxide [\[Al{{(-O-C{{(C{{H}_{3}})}_{3}})}_{3}}\]]
(ii) Dehydrogenation of \[{{\mathbf{1}}^{\mathbf{o}}}\] and \[{{\mathbf{2}}^{\mathbf{o}}}\] alcohols by \[\mathbf{Cu/30}{{\mathbf{0}}^{\mathbf{o}}}\]or \[\mathbf{Ag/30}{{\mathbf{0}}^{\mathbf{o}}}\mathbf{C}\].
\[R-C{{H}_{2}}OH\xrightarrow{Cu/\text{300}{}^\circ C}\overset{O}{\mathop{\overset{||}{\mathop{R-C-H}}\,}}\,+{{H}_{2}}\]
\[\overset{\,OH}{\mathop{\overset{|\ \ \ \ }{\mathop{R-CH-R'}}\,}}\,\xrightarrow{Cu/\text{300}{}^\circ C}\overset{O}{\mathop{\overset{||}{\mathop{R-C-R'}}\,\ }}\,+{{H}_{2}}\]
(2) From carboxylic acids
(i) Distillation of Ca, Ba, Sr or Th salts of monobasic acids
\[{{(RCOO)}_{2}}Ca+{{(R'COO)}_{2}}Ca\xrightarrow{\Delta }\overset{\,\,\,O}{\mathop{\overset{\,\,\,||}{\mathop{2R-C-R'}}\,}}\,+2CaC{{O}_{3}}\]
Thus in the product, one alkyl group comes from one carboxylic acid and other alkyl group from other carboxylic acid.
Calcium salts of dibasic acid (1, 4 and higher) on distillation give cyclic ketones.
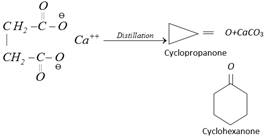
\[\left[ \overset{}{\mathop{O}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-{{(C{{H}_{2}})}_{5}}-\overset{\,\,\,\,\,\,\,\,\,}{\mathop{COO}}\, \right]C{{a}^{++}}\ \xrightarrow{\text{Distillation}}\]
(ii) Decarboxylation or Dehydration of acids by \[\mathbf{MnO/30}{{\mathbf{0}}^{\mathbf{o}}}\mathbf{C}\].
(a) This reaction takes place between two molecules of carboxylic acids. Both may be the same or different.
(b) If one of the carboxylic acids is \[HCOOH\] then this acid undergoes decarboxylation because this acid is the only monobasic acid which undergoes decarboxylation even in the absence of catalyst.
Case I : When both molecules are \[HCOOH\]
![]()
Case II : When only one molecule is formic acid.
![]()
Case III : When none of the molecule is formic acid.
![]()
(3) From gem dihalides : Gem dihalides on hydrolysis give carbonyl compounds
(i) \[\underset{\text{Gemdihalide}}{\mathop{R-CH{{X}_{2}}}}\,\xrightarrow{HOH/O\overset{}{\mathop{H}}\,}\underset{\text{Aldehyde}}{\mathop{R-CHO}}\,\]
(ii) \[R\underset{X}{\overset{X}{\mathop{\underset{|}{\overset{|}{\mathop{-C-}}}\,}}}\,R'\ \xrightarrow{HOH/O\overset{}{\mathop{H}}\,}R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,R'\]
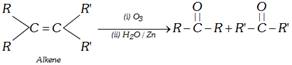
(4) From alkenes
(i) Ozonolysis : Alkenes on reductive ozonolysis give carbonyl compounds
\[\underset{\text{Alkene}}{\mathop{R-CH=CH-R}}\,\underset{\text{(ii)}\,{{H}_{2}}O/Zn}{\mathop{\xrightarrow{\text{(i)}\,{{O}_{3}}}}}\,R-CHO+RCHO\]
(ii) Oxo process
\[R-CH=C{{H}_{2}}+CO+{{H}_{2}}\overset{C{{O}_{2}}{{(CO)}_{8}}}{\mathop{\xrightarrow[150{}^\circ C,\ 300\ atm]{}}}\,\ R-C{{H}_{2}}-C{{H}_{2}}-CHO\]
(iii) Wacker process
(a) \[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\underset{\text{air}/C{{u}_{2}}C{{l}_{2}}}{\mathop{\xrightarrow{PdC{{l}_{2}}/HOH}}}\,C{{H}_{3}}-CHO\]
(b) \[\underset{\text{Alkyl ethene}}{\mathop{R-CH=C{{H}_{2}}}}\,\underset{\text{air}/C{{u}_{2}}C{{l}_{2}}}{\mathop{\xrightarrow{PdC{{l}_{2}}/HOH}}}\,R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{3}}\]
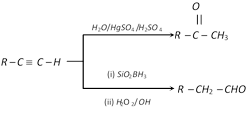
(5) From alkynes
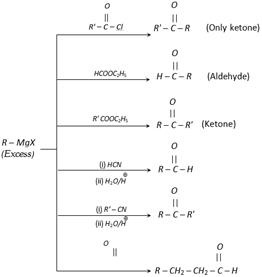
(6) From Grignard reagents
(7) From acid chloride
(i) Acid chlorides give nucleophilic substitution reaction with dialkyl cadmium and dialkyl lithium cuprate to give ketones. This is one of the most important method for the preparation of ketones from acid chlorides.
\[R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,Cl\ \ \xrightarrow{R{{'}_{2}}Cd}\ R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,R'\]
\[R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,Cl\ \ \xrightarrow{R{{'}_{2}}CuLi}\ R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,R'\]
(Only used for the preparation of ketones)
In this method product is always ketone because \[R\ne H\] and also \[R'\ne H\].
(ii) Rosenmunds reduction : This reduction takes place in the presence of Lindlars catalyst.
\[R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,Cl\underset{\text{Xylene}}{\mathop{\xrightarrow{{{H}_{2}}/Pd-BaS{{O}_{4}}-CaC{{O}_{3}}}}}\,\ R\overset{O}{\mathop{\overset{\,||}{\mathop{-\,C-}}\,}}\,H\]
\[Ar\overset{O}{\mathop{\overset{\,||}{\mathop{-C-}}\,}}\,Cl\underset{\text{Xylene}}{\mathop{\xrightarrow{{{H}_{2}}/Pd-BaS{{O}_{4}}-CaC{{O}_{3}}}}}\,\ Ar\overset{O}{\mathop{\overset{\,||}{\mathop{-\,C-}}\,}}\,H\]
(Only used for aldehydes)
(8) From cyanides
(i) Stephen aldehyde synthesis : Conversion of cyanides into aldehydes by partial reduction with \[SnC{{l}_{2}}/HCl\], followed by hydrolysis, is known as Stephens aldehyde synthesis.
\[\underset{\text{Alkyl cyanide}}{\mathop{R-C\equiv N}}\,\underset{\text{(ii) }{{H}_{2}}O/\Delta \text{ or steam distillation}}{\mathop{\xrightarrow{\,\,\,\,\,\,\text{(i) }SnC{{l}_{2}}/HCl/\text{ether}\,\,\,\,\,\,\,}}}\,\ \ \underset{\text{Aldehyde}}{\mathop{R-CHO}}\,\]
(Only used for aldehydes)
(9) From vic diols
\[R\overset{\,\,\,\,OH}{\mathop{\overset{|}{\mathop{-CH}}\,}}\,\underset{R\,\,\,}{\overset{\,OH}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-R}}}\,\xrightarrow{HI{{O}_{4}}}RCHO\ +\ R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,R+{{H}_{2}}O\]
(10) From Alkyl halides and benzyl halides
\[R-C{{H}_{2}}Cl\ \xrightarrow{DMSO}\ R-CHO\] ;
\[R\overset{Cl\,\,}{\mathop{-\overset{|}{\mathop{C}}\,H-}}\,R\ \xrightarrow{\text{DMSO}}\ \ R\overset{\,\,\,O\,\,}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,R\]
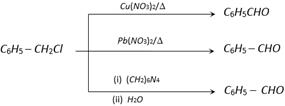
\[{{C}_{6}}{{H}_{5}}-C{{H}_{2}}Cl\overset{\text{DMSO}\ \text{or (i)}\ {{(C{{H}_{2}})}_{6}}{{N}_{4}}}{\mathop{\ \xrightarrow[(ii)\ {{H}_{2}}O/{{H}^{\oplus }}\ \text{or }Cu{{(N{{O}_{3}})}_{2}}\ \text{or }Pb{{(N{{O}_{3}})}_{2}}]{}}}\,\ {{C}_{6}}{{H}_{5}}-CHO\]
(11) From nitro alkanes : Nitro alkanes having at least one \[\alpha -\]hydrogen atom give carbonyl compounds on treatment with conc NaOH followed by 70% \[{{H}_{2}}S{{O}_{4}}\]. The reaction is known as Nef carbonyl synthesis.
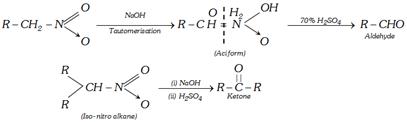
(12) Reaction with excess of alkyl lithium : Carboxylic acids react with excess of organo lithium compound to give lithium salt of gem diols which on hydrolysis give ketones.
\[R'\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,OH\ \ \underset{\text{(ii) }HOH/{{H}^{\oplus }}}{\mathop{\xrightarrow{\text{(i) }R-Li\ \text{(excess)}}}}\,\ R'\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,R\]
Preparation of only aromatic carbonyl compounds
(1) From methyl arenes

(2) From chloro methyl
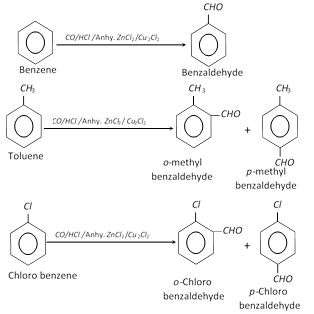
(3) Gattermann – Koch formylation : This reaction is mainly given by aromatic hydrocarbons and halobenzenes.
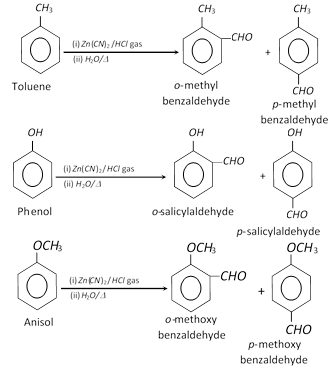
(4) Gattermann formylation : This reaction is mainly given by alkyl benzenes, phenols and phenolic ethers.
(5) Houben – Hoesch reaction : This reaction is given by di and polyhydric benzenes.
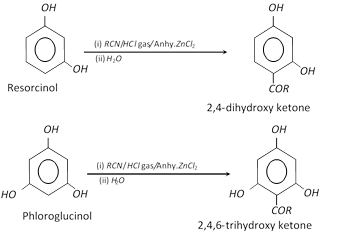
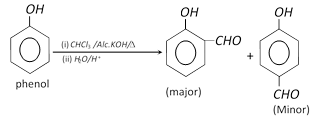
(6) Reimer – Tiemann reaction : Phenol gives o- and p- hydroxy benzaldehyde in this reaction.
You need to login to perform this action.
You will be redirected in
3 sec
