Polysaccharide (Starch and cellulose)
Category : JEE Main & Advanced
Polysaccharides are polymer of monosaccharide. The most important polysaccharides are starch and cellulose. They have a general formula \[{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}.\] Starch (Amylum) is most widely distributed in vegetable kingdom. It is found in the leaves, stems, fruits, roots and seeds. Concentrated form of starch is present in wheat, corn, barley, rice, potatoes, nuts, etc. It is the most important food source of carbohydrates.
(1) Starch and its derivatives : Starch is a white amorphous substance with no taste or smell. When heated to a temperature between \[200-{{250}^{o}}C,\] it changes into dextrin. At higher temperature charring occurs. When boiled with dilute acid, starch ultimately yields glucose.
\[\underset{\text{Starch}}{\mathop{{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}}}\,\xrightarrow{{}}\underset{\text{Dextrin}}{\mathop{{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{{{n}_{1}}}}}}\,\xrightarrow{{}}\]\[\underset{\text{Maltose}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,\xrightarrow{{}}\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
Both \[n\] and \[{{n}_{1}},\] are unknown, but \[n\] is believed to be greater than \[{{n}_{1}}\].
When treated with enzyme, diastase, it yields maltose.
\[2{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}+n{{H}_{2}}O\xrightarrow{{}}\underset{\text{Maltose}}{\mathop{n{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,\]
Starch solution gives a blue colour with a drop of iodine which disappears on heating to \[75-{{80}^{o}}C\] and reappears on cooling. The exact chemical nature of starch varies from source to source. Even the starch obtained from same source consists of two fractions
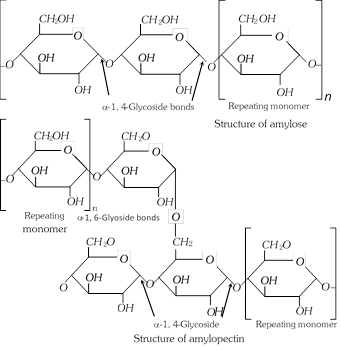
(i) amylose and
(ii) amylopectin.
Amylose is a linear polymer while amylopectin is a highly branched polymer. Both are composed of \[\alpha -D-\]glucose units linked by glycosidic linkages. The number of D-glucose units in amylose range from 60 – 300. It is soluble in hot water, Amylopectin consists of D-glucose units from 300 – 600. It is insoluble in water.
Uses : Starch and its derivatives are used
(i) As the most valuable constituent of food as rice, bread, potato and corn-flour, etc.
(ii) In the manufacture of glucose, dextrin and adhesives (starch paste).
(iii) In paper and textile industry.
(iv) In calico printing as a thickening agent for colours.
(v) Nitro starch is used as an explosive.
(vi) Starch-acetate is a transparent gelatin like mass and is used mainly for making sweets.
(2) Cellulose : It is found in all plants and so is the most abundant of all carbohydrates. It is the material used to form cell walls and other structural features of the plants. Wood is about 50% cellulose and the rest is lignin. Cotton and paper are largely composed of cellulose.
Pure cellulose is obtained by successively treating cotton, wool, flax or paper with dilute alkali, dilute \[HCl\] or \[HF\]. This treatment removes mineral matter, water, alcohol and ether. Cellulose is left behind as a white amorphous powder.
Cellulose is insoluble in water and in most of the organic solvents. It decomposes on heating but does not melt. It dissolves in ammonical copper hydroxide solution (Schwitzer’s reagent). Cellulose also dissolves in a solution of zinc chloride in hydrochloric acid.
When it is treated with concentrated \[{{H}_{2}}S{{O}_{4}}\] in cold, it slowly passes into solution. The solution when diluted with water, a starch like substance amyloid is precipitated and is called parchment paper. When boiled with dilute \[{{H}_{2}}S{{O}_{4}}\], it is completely hydrolysed into D-glucose.
\[\underset{\text{Cellulose}}{\mathop{{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}}}\,+n{{H}_{2}}O\xrightarrow{{}}\underset{\text{Glucose}}{\mathop{n{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
The cattle, goats and other ruminants can feed directly cellulose (grass, straw, etc.) as they have digestive enzymes (celluloses) capable of hydrolysing cellulose into glucose. Man and many other mammals lack the necessary enzymes in their digestive tract and thus cannot use cellulose as food stuff.
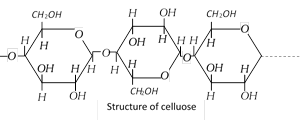
Cellulose is a straight chain polysaccharide composed of D-glucose units which are joined by B-glycosidic linkages between \[C-1\] of one glucose unit and \[C-4\] of the next glucose unit. The number of D-glucose units in cellulose ranges from 300 to 50000.
Uses : Cellulose is used
(i) As such in the manufacture of cloth (cotton), canvas and gunny bags (jute) and paper (wood, bamboo, straw, etc.)
(ii) In the form of cellulose nitrates for the manufacture of explosives (gun-powder), medicines, paints and lacquers. The cellulose nitrates with camphor yield celluloid which is used in the manufacture of toys, decorative articles and photographic films.
(iii) In the form of cellulose acetate for the manufacture of rayon (artificial silk) and plastics.
Distinction between glucose, sucrose, starch
| Test | Glucose | Sucrose | Starch |
| With iodine solution | No effect | No effect | Blue colour |
| With Fehling’s solution | Gives red precipitate | No effect | No effect |
| With Tollen’s reagent | Gives silver mirror | No effect | No effect |
| With phenyl hydrazine | Forms yellow osazone | No effect | No effect |
| Solubility in water | Soluble | Soluble | Insoluble |
| Taste | Sweet | Sweet | No taste |
You need to login to perform this action.
You will be redirected in
3 sec
