Acidic Nature Of Monocarboxylic Acids
Category : JEE Main & Advanced
(1) Cause of acidic nature
(i) A molecule of carboxylic acid can be represented as a resonance hybrid of the following structures.
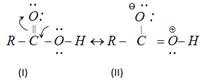
(ii) Due to electron deficiency on oxygen atom of the hydroxyl group (Structure II), their is a displacement of electron pair of O?H bond toward the oxygen atom. This facilitate the release of hydrogen as proton (H+).
![]()
(iii) The resulting carboxylate ion also stabilized by resonance (As negative charge is dispersed on both the oxygen atom). This enhance the stability of carboxylate anion and make it weaker base or strong acid.
(2) Effect of substituent on acidic nature
(i) An electron withdrawing substituent (– I effect) stabilizes the anion by dispersing the negative charge and therefore increases the acidity.
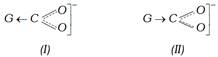
(ii) An electron releasing substituent (+ I effect) stabilizes negative charge on the anion resulting in the decrease of stability and thus decreased the acidity of acid.
Electron with drawing nature of halogen : F > Cl > Br > I
Thus, the acidic strength decreases in the order :
\[FC{{H}_{2}}COOH>ClC{{H}_{2}}COOH>BrC{{H}_{2}}COOH>IC{{H}_{2}}COOH\]
similarly :
\[CC{{l}_{3}}COOH>CHC{{l}_{2}}COOH>C{{H}_{2}}ClCOOH>C{{H}_{3}}COOH\]
(iii) Inductive effect is stronger at \[\alpha -\]position than \[\beta -\]position similarly at \[\beta -\]position it is more stronger than at \[\gamma -\]position
Example:
\[C{{H}_{3}}-C{{H}_{2}}-\underset{Cl\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-COOH>C{{H}_{3}}-\underset{Cl\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}-COOH\] \[>\underset{Cl\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-C{{H}_{2}}-C{{H}_{2}}-COOH\]
(iv) Relative acid strength in different compounds
\[RCOOH>HOH>ROH>HC\equiv CH>N{{H}_{3}}>RH\]
\[\begin{matrix} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,HCOOH\,\,\,\,\,\,\,>\,\,\,\,\,C{{H}_{3}}COOH>{{C}_{2}}{{H}_{5}}COOH \\ \begin{matrix} {{K}_{a}}\,\text{Value} & {} \\ \end{matrix}17.7\times {{10}^{-5}}\,\,\,\,\,\,\,\,\,\,\,\,\,1.75\times {{10}^{-5}}\,\,\,\,\,\,\,\,\,\,1.3\times {{10}^{-5}} \\ \end{matrix}\]
You need to login to perform this action.
You will be redirected in
3 sec
