Chemical Properties Of Monocarboxylic Acids
Category : JEE Main & Advanced
(1) Reaction involving removal of proton from \[-OH\] group
(i) Action with blue litmus : All carboxylic acids turn blue litmus red.
(ii) Reaction with metals
\[2C{{H}_{3}}COOH+2Na\to \underset{\text{Sodium acetate}}{\mathop{2C{{H}_{3}}COONa}}\,+{{H}_{2}}\]
\[2C{{H}_{3}}COOH+Zn\to (\underset{\text{Zinc acetate}}{\mathop{C{{H}_{3}}COO{{)}_{2}}}}\,Zn+{{H}_{2}}\]
(iii) Action with alkalies
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+NaOH\to \underset{\text{Sodium acetate}}{\mathop{C{{H}_{3}}COONa}}\,+{{H}_{2}}O\]
(iv) Action with carbonates and bicarbonates
\[2C{{H}_{3}}COOH+N{{a}_{2}}C{{O}_{3}}\to \underset{\text{Sod}\text{. acetate}}{\mathop{2C{{H}_{3}}COONa}}\,+C{{O}_{2}}+{{H}_{2}}O\]
\[C{{H}_{3}}COOH+NaHC{{O}_{3}}\to \underset{\text{Sod}\text{. acetate}}{\mathop{C{{H}_{3}}COONa}}\,+C{{O}_{2}}+{{H}_{2}}O\]
(2) Reaction involving replacement of –OH group
(i) Formation of acid chloride
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+PC{{l}_{5}}\to \underset{\text{Acetyl chloride}}{\mathop{3C{{H}_{3}}COCl}}\,+POC{{l}_{3}}+HCl\]
\[\underset{\text{Acetic acid}}{\mathop{3C{{H}_{3}}COOH}}\,+PC{{l}_{3}}\to \underset{\text{Acetyl chloride}}{\mathop{3C{{H}_{3}}COCl}}\,+{{H}_{3}}P{{O}_{3}}\]
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+SOC{{l}_{2}}\to \underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}COCl}}\,+S{{O}_{2}}+HCl\]
(ii) Formation of esters (Esterification)
![]()
\[\underset{\begin{smallmatrix} \text{Ethyl acetate} \\ \text{(Fruity smelling)}\end{smallmatrix}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+{{H}_{2}}O\]
(a) The reaction is shifted to the right by using excess of alcohol or removal of water by distillation.
(b) The reactivity of alcohol towards esterification.
tert-alcohol < sec-alcohol < pri-alcohol < methyl alcohol
(c) The acidic strength of carboxylic acid plays only a minor role.
\[{{R}_{3}}CCOOH<{{R}_{2}}CHCOOH<RC{{H}_{2}}COOH<C{{H}_{3}}COOH<HCOOH\]
When methanol is taken in place of ethanol. then reaction is called trans esterification.
(iv) Formation of amides
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+N{{H}_{3}}\xrightarrow{\text{heat}}\underset{\text{Amm}\text{. acetate}}{\mathop{C{{H}_{3}}COON{{H}_{4}}}}\,\xrightarrow{\Delta }\]
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+{{H}_{2}}O\]
(v) Formation of acid anhydrides
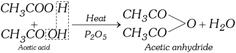
(vi) Reaction with organo-metallic reagents
\[R'C{{H}_{2}}MgBr+RCOOH\xrightarrow{\text{ether}}\underset{\text{Alkane}}{\mathop{R'C{{H}_{3}}}}\,+RCOOMgBr\]
(3) Reaction involving carbonyl \[(>C=O)\] group:
Reduction : \[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{LiAl{{H}_{4}}}R-C{{H}_{2}}-OH\]
Carboxylic acid are difficult to reduce either by catalytic hydrogenation or \[{Na}/{{{C}_{2}}{{H}_{5}}OH}\;\]
(4) Reaction involving attack of carboxylic group \[(-COOH)\]
(i) Decarboxylation : \[R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{(-C{{O}_{2}})}R-H\]
When anhydrous alkali salt of fatty acid is heated with sodalime then :
\[\underset{\text{Sodium salt}}{\mathop{RCOONa}}\,+NaOH\underset{\text{heat}}{\mathop{\xrightarrow{CaO}}}\,\underset{\text{Alkane}}{\mathop{R-H}}\,+N{{a}_{2}}C{{O}_{3}}\]
\[HCOONa+NaOH\xrightarrow{CaO}{{H}_{2}}+N{{a}_{2}}C{{O}_{3}}\]
(ii) Heating of calcium salts
\[\underset{\text{Sodium salt}}{\mathop{{{(RCOO)}_{2}}Ca}}\,\xrightarrow{\text{heat}}\underset{\text{Ketone}}{\mathop{RCOR}}\,+CaC{{O}_{3}}\]
(iii) Electrolysis : (Kolbe's synthesis)
\[RCOONa\] ? \[RCO{{O}^{-}}+N{{a}^{+}}\]
At anode \[2RCO{{O}^{-}}\to R-R+2C{{O}_{2}}+2{{e}^{-}}\]
At cathode \[2N{{a}^{+}}+2{{e}^{-}}\to 2Na\xrightarrow{2{{H}_{2}}O}2NaOH+{{H}_{2}}\]
\[\underset{\text{Potassium acetate}}{\mathop{2C{{H}_{3}}COOK}}\,+2{{H}_{2}}O\xrightarrow{\text{Electrolysis}}\]
\[\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,+2C{{O}_{2}}+2KOH+{{H}_{2}}\]
(iv) Formation of Alkyl halide (Hunsdiecker's reaction)
\[\underset{\text{Silver acetate}}{\mathop{C{{H}_{3}}COOAg}}\,+B{{r}_{2}}\underset{CC{{l}_{4}}}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\text{Methyl bromide}}{\mathop{C{{H}_{3}}Br}}\,+AgBr+C{{O}_{2}}\]
(v) Formation of amines (Schmidt reaction)
\[\underset{\text{Acid}}{\mathop{RCOOH}}\,+\underset{\begin{smallmatrix} \text{Hydrazoic} \\ \text{acid}\end{smallmatrix}}{\mathop{{{N}_{3}}H}}\,\xrightarrow{{{H}_{2}}S{{O}_{4}}(conc.)}\underset{\begin{smallmatrix} \text{Primary} \\ \text{amine}\end{smallmatrix}}{\mathop{RN{{H}_{2}}}}\,+C{{O}_{2}}+{{N}_{2}}\]
In Schmidt reaction, one carbon less product is formed.
(vi) Complete reduction
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+6HI\xrightarrow{P}\underset{\text{Ethane}}{\mathop{C{{H}_{3}}C{{H}_{3}}}}\,+2{{H}_{2}}O+3{{I}_{2}}\]
In the above reaction, the – COOH group is reduced to a \[C{{H}_{3}}\] group.
(5) Reaction involving hydrogen of a-carbon
Halogenation
(i) In presence of U.V. light
\[-\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-COOH+C{{l}_{2}}\xrightarrow{U.V.\Delta }\underset{\alpha \text{-chloro acid}}{\mathop{-\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-}}\,COOH+}}\,HCl\]
(ii) In presence of Red P and diffused light [Hell Volhard-zelinsky reaction]
Carboxylic acid having an a-hydrogen react with \[C{{l}_{2}}\] or \[B{{r}_{2}}\] in the presence of a small amount of red phosphorus to give chloro acetic acid. The reaction is known as Hell Volhard-zelinsky reaction.
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}},\text{red }{{P}_{4}}}}}\,\underset{\text{Chloro acetic acid}}{\mathop{ClC{{H}_{2}}COOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}},\text{red}\,{{P}_{4}}}}}\,\]
\[\underset{\text{Dichloro acetic acid}}{\mathop{C{{l}_{2}}CHCOOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}}\text{, red }{{P}_{4}}}}}\,\underset{\text{Trichloro acetic acid}}{\mathop{C{{l}_{3}}CCOOH}}\,\]
(a) The reaction is shifted to the right by using excess of alcohol or removal of water by distillation.
(b) The reactivity of alcohol towards esterification. tert-alcohol < sec-alcohol < pri-alcohol < methyl alcohol (c) The acidic strength of carboxylic acid plays only a minor role. ![]() When methanol is taken in place of ethanol. then reaction is called trans esterification. (iv) Formation of amides \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+N{{H}_{3}}\xrightarrow{\text{heat}}\underset{\text{Amm}\text{. acetate}}{\mathop{C{{H}_{3}}COON{{H}_{4}}}}\,\xrightarrow{\Delta }\] \[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+{{H}_{2}}O\] (v) Formation of acid anhydrides (vi) Reaction with organo-metallic reagents \[R'C{{H}_{2}}MgBr+RCOOH\xrightarrow{\text{ether}}\underset{\text{Alkane}}{\mathop{R'C{{H}_{3}}}}\,+RCOOMgBr\] (3) Reaction involving carbonyl (>C = O) group: Reduction :
When methanol is taken in place of ethanol. then reaction is called trans esterification. (iv) Formation of amides \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+N{{H}_{3}}\xrightarrow{\text{heat}}\underset{\text{Amm}\text{. acetate}}{\mathop{C{{H}_{3}}COON{{H}_{4}}}}\,\xrightarrow{\Delta }\] \[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+{{H}_{2}}O\] (v) Formation of acid anhydrides (vi) Reaction with organo-metallic reagents \[R'C{{H}_{2}}MgBr+RCOOH\xrightarrow{\text{ether}}\underset{\text{Alkane}}{\mathop{R'C{{H}_{3}}}}\,+RCOOMgBr\] (3) Reaction involving carbonyl (>C = O) group: Reduction : ![]() Carboxylic acid are difficult to reduce either by catalytic hydrogenation or \[{Na}/{{{C}_{2}}{{H}_{5}}OH}\;\] (4) Reaction involving attack of carboxylic group (? COOH) (i) Decarboxylation : \[R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{(-C{{O}_{2}})}R-H\] When anhydrous alkali salt of fatty acid is heated with sodalime then : \[\underset{\text{Sodium salt}}{\mathop{RCOONa}}\,+NaOH\underset{\text{heat}}{\mathop{\xrightarrow{CaO}}}\,\underset{\text{Alkane}}{\mathop{R-H}}\,+N{{a}_{2}}C{{O}_{3}}\] q When sodium formate is heated with sodalime H2 is evolved. (Exception) \[HCOONa+NaOH\xrightarrow{CaO}{{H}_{2}}+N{{a}_{2}}C{{O}_{3}}\] (ii) Heating of calcium salts \[\underset{\text{Sodium salt}}{\mathop{{{(RCOO)}_{2}}Ca}}\,\xrightarrow{\text{heat}}\underset{\text{Ketone}}{\mathop{RCOR}}\,+CaC{{O}_{3}}\] (iii) Electrolysis : (Kolbe's synthesis) \[RCOONa\rightleftharpoons RCO{{O}^{-}}+N{{a}^{+}}\] At anode \[2RCO{{O}^{-}}\to R-R+2C{{O}_{2}}+2{{e}^{-}}\] At cathode \[2N{{a}^{+}}+2{{e}^{-}}\to 2Na\xrightarrow{2{{H}_{2}}O}2NaOH+{{H}_{2}}\] \[\underset{\text{Potassium acetate}}{\mathop{2C{{H}_{3}}COOK}}\,+2{{H}_{2}}O\xrightarrow{\text{Electrolysis}}\] \[\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,+2C{{O}_{2}}+2KOH+{{H}_{2}}\] (iv) Formation of Alkyl halide (Hunsdiecker's reaction) \[\underset{\text{Silver acetate}}{\mathop{C{{H}_{3}}COOAg}}\,+B{{r}_{2}}\underset{CC{{l}_{4}}}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\text{Methyl bromide}}{\mathop{C{{H}_{3}}Br}}\,+AgBr+C{{O}_{2}}\] q In Hunsdiecker reaction, one carbon atom less alkyl halide is formed from acid salt. (v) Formation of amines (Schmidt reaction) \[\underset{\text{Acid}}{\mathop{RCOOH}}\,+\underset{\begin{smallmatrix} \text{Hydrazoic} \\ \text{acid} \end{smallmatrix}}{\mathop{{{N}_{3}}H}}\,\xrightarrow{{{H}_{2}}S{{O}_{4}}(conc.)}\underset{\begin{smallmatrix} \text{Primary} \\ \text{amine} \end{smallmatrix}}{\mathop{RN{{H}_{2}}}}\,+C{{O}_{2}}+{{N}_{2}}\] In Schmidt reaction, one carbon less product is formed. (vi) Complete reduction \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+6HI\xrightarrow{P}\underset{\text{Ethane}}{\mathop{C{{H}_{3}}C{{H}_{3}}}}\,+2{{H}_{2}}O+3{{I}_{2}}\] In the above reaction, the ? COOH group is reduced to a \[C{{H}_{3}}\] group. (5) Reaction involving hydrogen of a-carbon Halogenation (i) In presence of U.V. light \[-\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-COOH+C{{l}_{2}}\xrightarrow{U.V.\Delta }\underset{\alpha \text{-chloro acid}}{\mathop{-\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-}}\,COOH+}}\,HCl\] (ii) In presence of Red P and diffused light [Hell Volhard-zelinsky reaction] Carboxylic acid having an a-hydrogen react with Cl2 or Br2 in the presence of a small amount of red phosphorus to give chloro acetic acid. The reaction is known as Hell Volhard-zelinsky reaction. \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}},\text{red }{{P}_{4}}}}}\,\underset{\text{Chloro acetic acid}}{\mathop{ClC{{H}_{2}}COOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}},\text{red}\,{{P}_{4}}}}}\,\] \[\underset{\text{Dichloro acetic acid}}{\mathop{C{{l}_{2}}CHCOOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}}\text{, red }{{P}_{4}}}}}\,\underset{\text{Trichloro acetic acid}}{\mathop{C{{l}_{3}}CCOOH}}\,\]
Carboxylic acid are difficult to reduce either by catalytic hydrogenation or \[{Na}/{{{C}_{2}}{{H}_{5}}OH}\;\] (4) Reaction involving attack of carboxylic group (? COOH) (i) Decarboxylation : \[R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH\xrightarrow{(-C{{O}_{2}})}R-H\] When anhydrous alkali salt of fatty acid is heated with sodalime then : \[\underset{\text{Sodium salt}}{\mathop{RCOONa}}\,+NaOH\underset{\text{heat}}{\mathop{\xrightarrow{CaO}}}\,\underset{\text{Alkane}}{\mathop{R-H}}\,+N{{a}_{2}}C{{O}_{3}}\] q When sodium formate is heated with sodalime H2 is evolved. (Exception) \[HCOONa+NaOH\xrightarrow{CaO}{{H}_{2}}+N{{a}_{2}}C{{O}_{3}}\] (ii) Heating of calcium salts \[\underset{\text{Sodium salt}}{\mathop{{{(RCOO)}_{2}}Ca}}\,\xrightarrow{\text{heat}}\underset{\text{Ketone}}{\mathop{RCOR}}\,+CaC{{O}_{3}}\] (iii) Electrolysis : (Kolbe's synthesis) \[RCOONa\rightleftharpoons RCO{{O}^{-}}+N{{a}^{+}}\] At anode \[2RCO{{O}^{-}}\to R-R+2C{{O}_{2}}+2{{e}^{-}}\] At cathode \[2N{{a}^{+}}+2{{e}^{-}}\to 2Na\xrightarrow{2{{H}_{2}}O}2NaOH+{{H}_{2}}\] \[\underset{\text{Potassium acetate}}{\mathop{2C{{H}_{3}}COOK}}\,+2{{H}_{2}}O\xrightarrow{\text{Electrolysis}}\] \[\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,+2C{{O}_{2}}+2KOH+{{H}_{2}}\] (iv) Formation of Alkyl halide (Hunsdiecker's reaction) \[\underset{\text{Silver acetate}}{\mathop{C{{H}_{3}}COOAg}}\,+B{{r}_{2}}\underset{CC{{l}_{4}}}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\text{Methyl bromide}}{\mathop{C{{H}_{3}}Br}}\,+AgBr+C{{O}_{2}}\] q In Hunsdiecker reaction, one carbon atom less alkyl halide is formed from acid salt. (v) Formation of amines (Schmidt reaction) \[\underset{\text{Acid}}{\mathop{RCOOH}}\,+\underset{\begin{smallmatrix} \text{Hydrazoic} \\ \text{acid} \end{smallmatrix}}{\mathop{{{N}_{3}}H}}\,\xrightarrow{{{H}_{2}}S{{O}_{4}}(conc.)}\underset{\begin{smallmatrix} \text{Primary} \\ \text{amine} \end{smallmatrix}}{\mathop{RN{{H}_{2}}}}\,+C{{O}_{2}}+{{N}_{2}}\] In Schmidt reaction, one carbon less product is formed. (vi) Complete reduction \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+6HI\xrightarrow{P}\underset{\text{Ethane}}{\mathop{C{{H}_{3}}C{{H}_{3}}}}\,+2{{H}_{2}}O+3{{I}_{2}}\] In the above reaction, the ? COOH group is reduced to a \[C{{H}_{3}}\] group. (5) Reaction involving hydrogen of a-carbon Halogenation (i) In presence of U.V. light \[-\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,-COOH+C{{l}_{2}}\xrightarrow{U.V.\Delta }\underset{\alpha \text{-chloro acid}}{\mathop{-\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-}}\,COOH+}}\,HCl\] (ii) In presence of Red P and diffused light [Hell Volhard-zelinsky reaction] Carboxylic acid having an a-hydrogen react with Cl2 or Br2 in the presence of a small amount of red phosphorus to give chloro acetic acid. The reaction is known as Hell Volhard-zelinsky reaction. \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}},\text{red }{{P}_{4}}}}}\,\underset{\text{Chloro acetic acid}}{\mathop{ClC{{H}_{2}}COOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}},\text{red}\,{{P}_{4}}}}}\,\] \[\underset{\text{Dichloro acetic acid}}{\mathop{C{{l}_{2}}CHCOOH}}\,\underset{-HCl}{\mathop{\xrightarrow{C{{l}_{2}}\text{, red }{{P}_{4}}}}}\,\underset{\text{Trichloro acetic acid}}{\mathop{C{{l}_{3}}CCOOH}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
