Substituted Carboxylic Acids
Category : JEE Main & Advanced
The compounds formed by the replacement of one or more hydrogen atoms of the hydrocarbon chain part of the carboxylic acids by atoms or groups such as X (halogen), OH or \[N{{H}_{2}},\] are referred to as substituted acids. For example,
\[\underset{\text{Chloroacetic acid}}{\mathop{C{{H}_{2}}ClCOOH}}\,\]; \[\underset{\text{Hydroxyacetic acid}}{\mathop{C{{H}_{2}}OHCOOH}}\,\]; \[\underset{\text{Aminoacetic acid}}{\mathop{C{{H}_{2}}N{{H}_{2}}COOH}}\,\]
The position of the substituents on the carbon chain are indicated by Greek letters or numbers.
\[\underset{\varepsilon }{\overset{6}{\mathop{C}}}\,-\underset{\delta }{\overset{5}{\mathop{C}}}\,-\underset{\gamma }{\overset{4}{\mathop{C}}}\,-\underset{\beta }{\overset{3}{\mathop{C}}}\,-\underset{\alpha }{\overset{2}{\mathop{C}}}\,-\overset{1}{\mathop{C}}\,OOH\]
For example,
\[\underset{\begin{smallmatrix} \alpha \text{-Hydroxypropionic acid} \\ \text{2-Hydroxypropanoic acid} \end{smallmatrix}} {\mathop{C{{H}_{3}}CHOHCOOH}}\,\]; \[\underset{\begin{smallmatrix} \beta \text{-Hydroxybutyric acid} \\ \text{3-Hydroxybutanoic acid} \end{smallmatrix}}{\mathop{C{{H}_{3}}CHOHC{{H}_{2}}COOH}}\,\]
Lactic Acid or a-hydroxy propionic acid or 2-hydroxy propanoic acid
It is the main constituent of sour milk. It is manufactured by fermentation of molasses by the micro-organism (Bacterium acidi lactici-sour milk) in presence of \[CaC{{O}_{3}}\].
(1) Method of Preparation
From acetaldehyde :
\[\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,+HCN\to \underset{\text{Cyanohydrin}}{\mathop{C{{H}_{3}}CH(OH)CN}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\] \[\underset{\text{Lactic acid}}{\mathop{C{{H}_{3}}CHOHCOOH}}\,\]
(2) Physical Properties
It is a colourless syrupy liquid having a sour taste and smell.
It is hygroscopic and very soluble in water. It is optically active and exists in three distinct forms.
(3) Chemical Properties : It gives reactions of secondary alcoholic group and a carboxylic group.
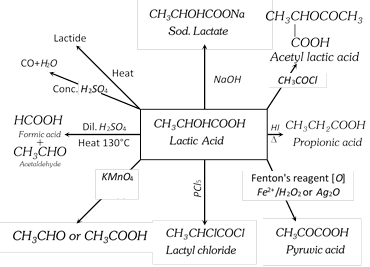
(4) Uses : It is used in medicine as calcium and iron lactates, as mordant in dyeing, as acidulant in beverages and candies, as a solvent (ethyl and butyl lactates) for cellulose nitrate.
Tartaric Acid. Or \[\alpha ,\,\,\alpha '-\]Dihydroxy succinic acid or 2, 3-Dihydroxy-Butane-1,4-Dioic acid
\[\underset{HO-CH-COOH}{\mathop{HO-\underset{|}{\mathop{C}}\,H-COOH}}\,\]
It is found as free or potassium salt in grapes, tamarind, and berries.
(1) Methods of Preparation
(i) Argol which separates as a crust during fermentation of grape juice is impure potassium hydrogen tartrate. Argol is boiled with limewater. Calcium tartrate is precipitated which is filtered. The solution contains potassium tartrate which is also precipitated by addition of \[CaC{{l}_{2}}\]. The calcium salt is then decomposed with calculated quantity of dilute \[{{H}_{2}}S{{O}_{4}}\]. The precipitate \[(CaS{{O}_{4}})\] is filtered and the filtrate on concentration gives the crystals of tartaric acid.
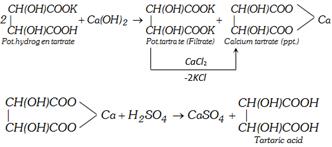
(ii) Synthetic method
\[C+{{H}_{2}}\underset{arc}{\mathop{\xrightarrow{\text{Electric}}}}\,\underset{\text{Acetylene}}{\mathop{CH\equiv CH}}\,\underset{{Pd}/{BaS{{O}_{4}}}\;}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\xrightarrow{B{{r}_{2}}}\]
\[\underset{\text{Ethylene bromide}}{\mathop{{{(C{{H}_{2}}Br)}_{2}}}}\,\xrightarrow{2KCN}\underset{C{{H}_{2}}CN}{\overset{C{{H}_{2}}CN}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\underset{\text{Succinic acid}}{\mathop{\underset{C{{H}_{2}}C{{O}_{2}}H}{\overset{C{{H}_{2}}C{{O}_{2}}H}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
\[\underset{B{{r}_{2}}}{\mathop{\xrightarrow{\operatorname{Re}\text{d}\,P}}}\,\underset{\begin{smallmatrix} \alpha \text{,}\alpha \text{ }\!\!'\!\!\text{ -Dibromo succinic} \\ \text{ acid} \end{smallmatrix}}{\mathop{\underset{CHBrCOOH}{\overset{CHBrCOOH} {\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\] \[\xrightarrow{AgOH}\underset{\text{Tartaric acid}}{\mathop{\underset{CHOHCOOH}{\overset{CHOHCOOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(iii) From glyoxal cyanohydrin :
\[\underset{\text{Glyoxal}}{\mathop{\underset{CHO}{\overset{CHO}{\mathop{|\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{HCN}\underset{\text{Glyoxal cyanohydrin}}{\mathop{\underset{CH(OH)CN}{\overset{CH(OH)CN}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\underset{\text{Tartaric acid}}{\mathop{\underset{CH(OH)COOH}{\overset{CH(OH)COOH}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(2) Physical Properties : It is a colourless crystalline compound. It is soluble in water and alcohol but insoluble in ether. It contains two asymmetric carbon atoms and thus shows optical isomerism (four forms). Natural tartaric acid is the dextro variety. It contains two secondary alcoholic groups and two carboxylic groups.
Optical Isomerism in tartaric acid
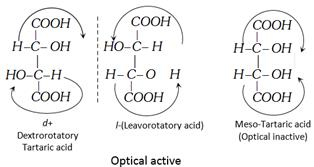
![]()
(i) d + Tartaric acid-Dextro-rotatory
(ii) l –Tartaric acid-Leavorotatory
(iii) Meso tartaric acid-optically inactive due to internal compensation.
(3) Chemical Properties
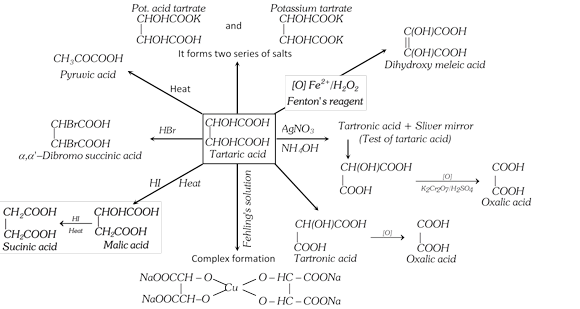
(4) Uses : It is used in carbonated beverages and effervescent tablets, in making baking powder (cream of tartar) and mordant in dyeing (potassium hydrogen tartrate), in preparing Fehling's solution (sodium potassium tartrate–Rochelle salt), in medicine as emetic, dyeing and calico-printing (tartar emetic-potassium antimonyl tartrate) and silver mirroring.
(5) Tests
(i) When heated strongly, tartaric acid chars readily giving a smell of burnt sugar to produce free carbon and pyruvic acid.
(ii) With \[AgN{{O}_{3}}\] : A neutral solution of tartaric acid gives a white ppt. which is soluble in ammonia. A silver mirror is obtained on warming the ammonical silver nitrate solution (Tollen's reagent).
(iii) With Fenton's reagent : (\[{{H}_{2}}{{O}_{2}}\] containing a little of ferrous salt) and caustic soda, It gives a violet colour.
(iv) With Resorcinol and conc. \[{{H}_{2}}S{{O}_{4}}\] : It gives blue colour.
Citric Acid Or 2-Hydroxypropane Or 1, 2, 3-Tri Carboxylic Acid Or \[\beta -\]Hydroxy Tricarballylic Acid
It occurs in the juice of citrus fruits such as lemon, galgal, orange, lime, etc. Lemon juice contains 6-10% of citric acid.
(1) Methods of Preparation
(i) By Fermentation : Citric acid is obtained by carrying fermentation of dilute solution of molasses with micro-organism, Aspergillus nigar, at \[26-{{28}^{o}}C\] for 7 to 10 days. The resulting solution is neutralised with \[Ca{{(OH)}_{2}}\] to form insoluble precipitate, calcium citrate. It is decomposed by dilute \[{{H}_{2}}S{{O}_{4}}\]. The \[CaS{{O}_{4}}\]is filtered off and the solution is concentrated under vacuum to get crystals of citric acid.
(ii) By Lemon juice : It is also obtained from lemon juice. The juice is boiled to coagulate proteins. From clear solution, citric acid is obtained as calcium salt with \[Ca{{(OH)}_{2}}\].
(iii) By synthetic method
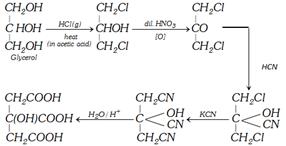
(2) Physical Properties : It is a colourless crystalline compound. It possesses one water molecule as water of crystallisation. It is soluble in water and alcohol but less soluble in ether. It is not optically active compound. It is nontoxic in nature. It behaves as an alcohol and tribasic acid.
(3) Chemical properties
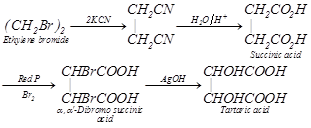
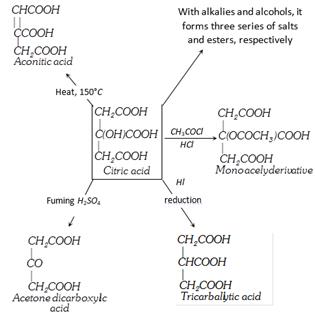
(4) Uses : It finds use in making lemonades, as acidulant in food and soft drinks and makes the lemon sour, as mordant in dyeing and calico printing. Ferric ammonium citrate, magnesium citrate (as an antacid and laxative), sodium or potassium citrate are used in medicine. Ferric ammonium citrate finds use in making blue prints.
You need to login to perform this action.
You will be redirected in
3 sec
