Covalent Bond
Category : JEE Main & Advanced
Covalent bond was first proposed by Lewis in 1916. The bond formed between the two atoms by mutual sharing of electrons so as to complete their octets or duplets (in case of elements having only one shell) is called covalent bond or covalent linkage. A covalent bond between two similar atoms is non-polar covalent bond while it is polar between two different atom having different electronegativities. Covalent bond may be single, double or a triple bond. We explain covalent bond formation by Lewis octet rule.
Chlorine atom has seven electrons in the valency shell. In the formation of chlorine molecule, each chlorine atom contributes one electron and the pair of electrons is shared between two atoms. both the atoms acquire stable configuration of argon.
\[\underset{(2,\,8,\,7)}{\mathop{_{\bullet }^{\bullet }\underset{\bullet \,\,\bullet }{\overset{\bullet \,\,\bullet }{\mathop{Cl}}}\,\,\bullet }}\,\,\underset{(2,\,8,\,7)}{\mathop{\,\,\,*\underset{*\,\,*}{\overset{*\,\,*}{\mathop{Cl\,_{*}^{*}}}}\,}}\,\,\,\,\,\,\to \,\,\,\,\underset{\,(2,\,8,\,8)\,\,\,\,\,\,\,(2,\,8,8)}{\mathop{\,\underset{\bullet \,\,\bullet \,\,}{\overset{\bullet \,\,\bullet \,\,}{\mathop{_{\bullet }^{\bullet }Cl\,\,_{\,*}^{\,\bullet }}}}\,\,\,\underset{*\,\,*\,\,\,\,\,}{\overset{*\,\,*\,\,\,\,\,}{\mathop{Cl\,_{*}^{*}}}}\,}}\,\] or \[Cl-Cl\]
Some other examples are : \[{{H}_{2}}S,N{{H}_{3}},HCN,PC{{l}_{3}},P{{H}_{3,}}\] \[{{C}_{2}}{{H}_{2}},{{H}_{2}},{{C}_{2}}{{H}_{4}},SnC{{l}_{4}},FeC{{l}_{3}},B{{H}_{3}},\]graphite, \[BeC{{l}_{2}}\]etc.
(1) Conditions for formation of covalent bond
(i) The combining atoms should be short by 1, 2 or 3 electrons in the valency shell in comparison to stable noble gas configuration.
(ii) Electronegativity difference between the two atoms should be zero or very small.
(iii) The approach of the atoms towards one another should be accompanied by decrease of energy.
(2) Characteristics of covalent compounds
(i) These exist as gases or liquids under the normal conditions of temperature and pressure. Some covalent compounds exist as soft solids.
(ii) Diamond, Carborandum (SiC), Silica (SiO2), AlN etc. have giant three dimensional network structures; therefore have exceptionally high melting points otherwise these compounds have relatively low melting and boiling points.
(iii) In general covalent substances are bad conductor of electricity. Polar covalent compounds like HCl in solution conduct electricity. Graphite can conduct electricity in solid state since electrons can pass from one layer to the other.
(iv) These compounds are generally insoluble in polar solvent like water but soluble in non-polar solvents like benzene etc. some covalent compounds like alcohol, dissolve in water due to hydrogen bonding.
(v) The covalent bond is rigid and directional. These compounds, thus show isomerism (structural and space).
(vi) Covalent substances show molecular reactions. The reaction rates are usually low.
(vii) The number of electrons contributed by an atom of the element for sharing with other atoms is called covalency of the element. Covalency = 8 – [Number of the group to which element belongs]. The variable covalency of an element is equal to the total number of unpaired electrons in s, p and d-orbitals of its valency shell.
The element such as P, S, Cl, Br, I have vacant d-orbitals in their valency shell. These elements show variable covalency by increasing the number of unpaired electrons under excited conditions. The electrons from paired orbitals get excited to vacant d-orbitals of the same shell.
Four elements, H, N, O and F do not possess d-orbitals in their valency shell. Thus, such an excitation is not possible and variable valency is not shown by these elements. This is reason that NCl3 exists while NCl5 does not.
(3) The Lewis theory : The tendency of atoms to achieve eight electrons in their outermost shell is known as lewis octet rule.
Lewis symbol for the representative elements are given in the following table,
|
|
1 |
2 |
13 |
14 |
15 |
16 |
17 |
|
|
Group |
IA |
IIA |
IIIA |
IVA |
VA |
VIA |
VIIA |
|
|
Lewis symbol |
X· |
·X· |
\[\bullet \overset{\bullet }{\mathop{X}}\,\,\bullet \] |
\[\bullet \overset{\bullet }{\mathop{\underset{\bullet }{\mathop{X}}\,}}\,\,\bullet \] |
\[\bullet \overset{\bullet }{\mathop{\underset{\bullet \ \bullet }{\mathop{X}}\,}}\,\,\bullet \] |
\[\bullet \overset{\bullet \ \bullet }{\mathop{\underset{\bullet \ \bullet }{\mathop{X}}\,}}\,\,\bullet \] |
\[_{\bullet }^{\bullet }\overset{\bullet \ \bullet }{\mathop{\underset{\bullet \ \bullet }{\mathop{X}}\,}}\,\,\bullet \]. |
|
(4) Failure of octet rule : There are several stable molecules known in which the octet rule is violated i.e., atoms in these molecules have number of electrons in the valency shell either short of octet or more than octet.
\[Be{{F}_{2}},B{{F}_{3}},Al{{H}_{3}}\] are electron- deficients (Octet incomplete) hence are Lewis acid.
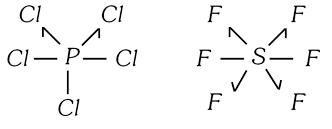
In \[PC{{l}_{5}},\ P\]has 10 electrons in valency shell while in \[S{{F}_{6}},\ S\] has 12 electrons in valence shell. Sugden introduced singlet linkage in which one electron is donated (Instead of one pair of electrons) to the electron deficient atom so that octet rule is not violated. This singlet is represented as (?). Thus, \[PC{{l}_{5}}\] and \[S{{F}_{6}}\]have structures as,
(5) Construction of structures for molecules and poly atomic ions : The following method is applicable to species in which the octet rule is not violated.
(i) Determine the total number of valence electrons in all the atoms present, including the net charge on the species (n1).
(ii) Determine n2 = [2 × (number of H atoms) + 8 × (number of other atoms)].
(iii) Determine the number of bonding electrons, n3, which equals n2 – n1. No. of bonds equals n3/2.
(iv) Determine the number of non-bonding electrons, n4, which equals n1 – n3. No. of lone pairs equals n4/2.
(v) Knowing the central atom (you’ll need to know some chemistry here, math will not help!), arrange and distribute other atoms and n3/2 bonds. Then complete octets using n4/2 lone pairs.
(vi) Determine the ‘formal charge’ on each atom.
(vii) Formal Charge = [valence electrons in atom) – (no. of bonds) – (no. of unshared electrons)]
(viii) Other aspects like resonance etc. can now be incorporated.
Illustrative examples
(i) \[CO_{3}^{2-};\,{{n}_{1}}=4+(6\times 3)+2=24\] [2 added for net charge]
\[{{n}_{2}}\] = (2 × 0) + (8 × 4) = 32 (no. H atom, 4 other atoms (1’C’ and 3 ‘O’)
\[{{n}_{3}}\] = 32 – 24 = 8, hence 8/2 = 4 bonds
\[{{n}_{4}}\] = 24 – 8 = 16, hence 8 lone pairs.
Since carbon is the central atom, 3 oxygen atoms are to be arranged around it, thus,
\[O-\overset{O}{\mathop{\overset{|\,}{\mathop{C\,}}\,}}\,-O\], but total bonds are equal to 4.
Hence, we get \[O-\overset{O}{\mathop{\overset{|}{\mathop{C}}\,}}\,=O\]. Now, arrange lone pairs to complete octet \[\overset{.\,}{\mathop{:\underset{.\,\,.}{\mathop{O}}\,}}\,\,\,-\overset{\overset{.\,\,.}{\mathop{.O:}}\,}{\mathop{\overset{|}{\mathop{C}}\,}}\,=\overset{.\,\,.}{\mathop{O}}\,:\]
(ii) \[C{{O}_{2}};\] n1 = 4 + (6 × 2 ) = 16
n2 = (2 × 0) + (8 × 3) = 24
n3 = 24 – 16 = 8, hence 4 bonds
n4 = 16 – 8 = 8, hence 4 lone-pairs
Since C is the central atom, the two oxygen atoms are around to be arranged it thus the structure would be; O – C – O, but total no. of bonds = 4
Thus, O = C = O. After arrangement of lone pairs to complete octets, we get, \[:\overset{.\,\,.}{\mathop{O}}\,=C=\overset{.\,\,.}{\mathop{O}}\,:\] and thus final structure is \[:\overset{.\,\,.}{\mathop{O}}\,=C=\overset{.\,\,.}{\mathop{O}}\,:\]
You need to login to perform this action.
You will be redirected in
3 sec
