Dipole moment
Category : JEE Main & Advanced
?The product of magnitude of negative or positive charge (q) and the distance (d) between the centres of positive and negative charges is called dipole moment?.
\[\mu =Electric\text{ }charge\times bond\text{ }length\]
As q is in the order of \[{{10}^{-10}}\] esu and d is in the order of \[{{10}^{-8}}\] cm, m is in the order of \[{{10}^{-18}}\] esu cm. Dipole moment is measured in "Debye" (D) unit. \[1D={{10}^{-18}}\] esu cm = \[3.33\times {{10}^{-30}}\] coulomb metre (In S.I. unit).
Dipole moment is indicated by an arrow having a symbol ![]() pointing towards the negative end. Dipole moment has both magnitude and direction and therefore it is a vector quantity.
pointing towards the negative end. Dipole moment has both magnitude and direction and therefore it is a vector quantity.
Symmetrical polyatomic molecules are not polar so they do not have any value of dipole moment. 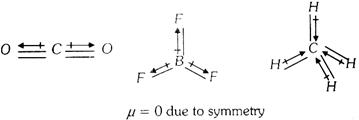
Unsymmetrical polyatomic molecules always have net value of dipole moment, thus such molecules are polar in nature. H2O, CH3Cl, NH3, etc are polar molecules as they have some positive values of dipole moments.
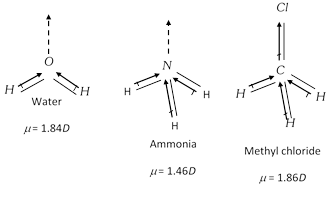
\[\mu =0\]due to unsymmetry
(1) Dipole moment is an important factor in determining the geometry of molecules. Molecular geometry and dipole moment
| General formula | Molecular geometry | Dipole moment | Example |
| AX | Linear | May be non zero | HF, HCl |
| |
Linear Bent or V-shape | Zero Non zero | |
| |
Triangular planar Pyramidal T-shape | Zero Non zero Non zero | |
| |
Tetrahedral Square planar See saw | Zero Zero Non zero | |
| |
Trigonal bipyramidal Square pyramidal | Zero Non zero | |
| |
Octahedral Distorted octahedral | Zero Non zero | |
| |
Pentagonal bipyramidal | Zero | |
(2) Every ionic compound having some percentage of covalent character according to Fajan's rule. The percentage of ionic character in compound having some covalent character can be calculated by the following equation.
The % ionic character ![]() .
.
(3) The trans isomer usually possesses either zero dipole moment or very low value in comparison to cis?form 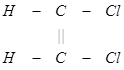
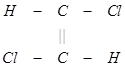
You need to login to perform this action.
You will be redirected in
3 sec
