Diagonal relationship
Category : JEE Main & Advanced
Certain elements of 2nd period show similarity with their diagonal elements in the 3rd period as shown below :
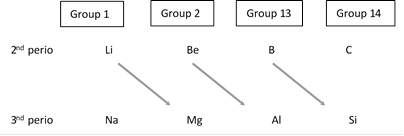
Thus, Li resembles Mg, Be resembles Al and B resembles Si. This is called diagonal relationship and is due to the reason that these pairs of element have almost identical ionic radii and polarizing power (i.e. charge/size ratio). Element of second period are known as bridge elements.
Anomalous behaviour of the first elements of a group: The first element of a group differs considerably from its congeners (i.e. the rest of the element of its group). This is due to (i) small size (ii) high electronegativity and (iii) non availability of d-orbitals for bonding. Anomalous behaviour is observed among the second row elements (i.e. Li to F).
You need to login to perform this action.
You will be redirected in
3 sec
