Addition reactions
Category : JEE Main & Advanced
These reactions are given by those compounds which have at least one \[\pi \] bond,
i.e., \[(>C=C<,-C\equiv C-,-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-,C\equiv N).\] In such reaction there is loss of one \[\pi \] bond and gain of two \[\sigma \] bonds. Thus product of the reaction is generally more stable than the reactant. The reaction is a spontaneous reaction.
Types of addition reactions : Addition reactions can be classified into three categories on the basis of the nature of initiating species.
(1) Electrophilic additions
(2) Nucleophilic additions
(3) Free radical additions
(1) Electrophilic addition reactions
(i) Such reactions are mainly given by alkenes and alkynes.
(ii) Electrophilic addition reactions of alkenes and alkynes are generally two step reactions.
(iii) Alkenes and alkynes give electrophilic addition with those reagents which on dissociation gives electrophile as well as nucleophile.
(iv) If the reagent is a weak acid then electrophilic addition is catalysed by strong acids (Generally \[{{H}_{2}}S{{O}_{4}}\]).
(v) Unsymmetrical alkenes and alkynes give addition reactions with unsymmetrical reagents according to Markownikoff?s rule.
The negative part of the addendum adds on that doubly bonded carbon of the alkene which has least number of hydrogen atom.
This rule can be used only in those alkenes which fulfil the following conditions:
(a) Alkene should be unsymmetrical.
(b) Substituent/substituents present on doubly bonded carbon/(s) should only be +I group.
(c) If phenyl group is present on doubly bonded carbon, then both doubly bonded carbons should be substituted by phenyl groups.
For example, the following alkenes will give addition according to the Markownikoff?s rule.
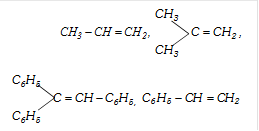
Following alkenes will not give addition reaction according to Markownikoff's rule
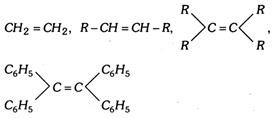
(vi) Unsymmetrical alkenes having the following general structure give addition according to anti Markownikoff's rule. \[C{{H}_{2}}=CH-G\], where G is a strong - I group such as
\[-C{{X}_{3}},-N{{O}_{2,}}-CN,-CHO,-COR,-COOH,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-Z\,\,\]\[(Z=Cl,OH,OR,N{{H}_{2}})\]
Example:
\[C{{H}_{2}}=CH-CHO+HCl\xrightarrow{\text{Anti-Markownikoff }\!\!'\!\!\text{ s addition}}{{\overset{Cl\,}{\mathop{\overset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,}_{2}}-C{{H}_{2}}-CHO\]
(vii) Mechanism of electrophilic addition reactions is as follows,
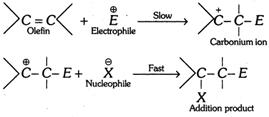
(2) Nucleophilic addition reactions : When the addition reaction occurs on account of the initial attack of nucleophile, the reaction is said to be a nucleophilic addition reaction. Due to presence of strongly electronegative oxygen atom, the \[\pi -\]electrons of the carbon-oxygen double bond in carbonyl group (\[C=O\]) get shifted towards the oxygen atom and thereby such bond is highly polarised. This makes carbon atom of the carbonyl group electron deficient.
![]()
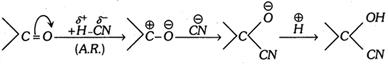
Example : The addition of HCN to acetone is an example of nucleophilic addition.
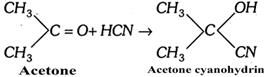
The mechanism of the reaction involves the following steps:
Step 1. HCN gives a proton \[(\overset{\oplus }{\mathop{H}}\,)\] and a nucleophile, cyanide ion \[(\overset{\Theta }{\mathop{CN}}\,)\].
\[HCN\to {{H}^{\oplus }}+C{{N}^{\Theta }}\]
Step 2. The nucleophile \[(C{{N}^{\Theta }})\] attacks the positively charged carbon so as to form an anion [\[{{H}^{\oplus }}\] does not initiate the negatively charged oxygen as anion is more stable than cation].
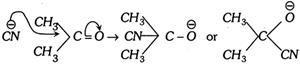
Step 3. The proton \[({{H}^{+}})\] combines with anion to form the addition product.
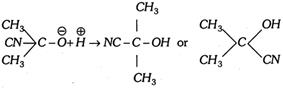
In \[\begin{matrix} {} \\ {} \\ \end{matrix}C=O\] compounds, the addition of liquid HCN gives cyanohydrin and the addendum is \[C{{N}^{-}}\] ion and not HCN directly (addition is catalysed by bases or salts of weak acids and retarded by acids or unaffected by neutral compounds).
Nucleophilic addition (AN) reactions on carbonyl compounds will be in order:
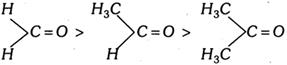
Decreasing order of nucleophilic addition in some species.
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}COC{{H}_{3}}>C{{H}_{3}}COC{{H}_{3}}>{{C}_{6}}{{H}_{5}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-C{{H}_{3}}>{{C}_{6}}{{H}_{5}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}-CHO>\] \[-COC{{H}_{3}}>-COCl>-COOC{{H}_{3}}>-CON{{H}_{2}}>-COOH\] \[\]
(3) Free radical addition reactions : Those reactions which involve the initial attack by a free radical are known as free radical reactions. Addition of hydrogen bromide to alkenes (say, propylene) in the presence of peroxide (radical initiator) follows free radical mechanism. Free radical reactions generally take place in non-polar solvents such as \[CC{{l}_{4}},\] high temperature, in presence of light or a free radical producing substance like \[{{O}_{2}}\] and peroxides.
You need to login to perform this action.
You will be redirected in
3 sec
