Water
Category : JEE Main & Advanced
Water is the oxide of hydrogen. It is an important component of animal and vegetable matter. Water constitutes about 65% of our body. It is the principal constituent of earth's surface.
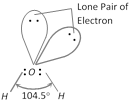
(1) Structure : Due to the presence of lone pairs, the geometry of water is distorted and the \[H-O-H\] bond angle is 104.5°, which is less than the normal tetrahedral angle (109.5°). The geometry of the molecule is regarded as angular or bent. In water, each \[O-H\]bond is polar because of the high electronegativity of oxygen (3.5) in comparison to that of hydrogen (2.1). The resultant dipole moment of water molecule is 1.84D.
In ice, each oxygen atom is tetrahedrally surrounded by four hydrogen atoms; two by covalent bonds and two by hydrogen bonds. The resulting structure of ice is open structure having a number of vacant spaces. Therefore, the density of ice is less than that of water and ice floats over water. It may be noted that water has maximum density \[(1g\,c{{m}^{-3}})\]at 4°C (277 K).
(2) Heavy water : Chemically heavy water is deuterium oxide\[({{D}_{2}}O)\]. It was discovered by Urey.
It is obtained as a by-product in some industries where \[{{H}_{2}}\] is produced by the electrolysis of water.
Heavy water \[({{D}_{2}}O)\] is used (a) as a moderator and coolant in nuclear reactors (b) in the study of mechanism of chemical reactions (c) as a starting material for the preparation of a number of deuterium compounds, e.g.,
\[S{{O}_{3}}+{{D}_{2}}O\to \underset{\text{Deuteriosulphuric acid}}{\mathop{{{D}_{2}}S{{O}_{4}}}}\,\]
\[A{{l}_{4}}{{C}_{3}}+12\,{{D}_{2}}O\to \underset{\text{Deuteromethane}}{\mathop{3C{{D}_{4}}}}\,+\ \ \ \ 4Al{{(OD)}_{3}}\]
\[Ca{{C}_{2}}+2{{D}_{2}}O\to \underset{\text{Deuterioa}\text{cetylene}}{\mathop{{{C}_{2}}{{D}_{2}}}}\,+\ \ \ Ca{{(OD)}_{2}}\]
(3) Physical properties : Water is colourless, odourless and tasteless liquid at ordinary temperature.
At 273K water is in equilibrium with ice and vapour this point is known triple point.
Some physical constants of H2O and D2O at 298 K
| Constant | Ordinary water H2O | Heavy water D2O |
| Molecular mass | 18.015 | 20.028 |
| Maximum density \[(g\,c{{m}^{-3}})\] | 1.000 | 1.106 |
| Melting point (K) | 273.2 | 276.8 |
| Boiling point (K) | 373.2 | 374.4 |
| Heat of fusion\[(kJ\,mo{{l}^{-1}})\]at 273K | 6.01 | 6.28 |
| Heat of vaporisation \[(kJ\,mo{{l}^{-1}})\]at 373K | 40.66 | 41.61 |
| Heat of formation \[(kJ\,mo{{l}^{-1}})\] | ? 285.9 | ? 294.6 |
| Ionisation constant | \[1.008\times {{10}^{-14}}\] | \[1.95\times {{10}^{-15}}\] |
(4) Chemical properties : Water shows a versatile chemical behaviour. It behaves as an acid, a base, an oxidant, a reductant and as ligand to metals.
(i) Dissociation of water : Water is quite stable and does not dissociate into its elements even at high temperatures. Pure water has a small but measurable electrical conductivity and it dissociates as,
\[{{H}_{2}}O+{{H}_{2}}O\]?\[\underset{Hydronium\,ion}{\mathop{{{H}_{3}}{{O}^{+}}}}\,+O{{H}^{-}}\]
\[{{K}_{W}}=1.0\times {{10}^{-14}}\,mo{{l}^{2}}{{L}^{2}}\]at 298K
(ii) Amphoteric nature : Water can act both as an acid and a base and is said to be amphoteric. However, water is neutral towards litmus and its pH is 7.
(iii) Oxidising and reducing nature : Water can act both as an oxidising and a reducing agent in its chemical reactions. e.g.
\[2Na+\underset{Oxidi\sin g\,agent}{\mathop{2{{H}_{2}}O}}\,\to 2NaOH+{{H}_{2}}\]
\[2{{F}_{2}}+\underset{\operatorname{Re}ducing\,agent}{\mathop{2{{H}_{2}}O}}\,\to 4HF+{{O}_{2}}\]
(iv) Hydrolytic reactions : Water can hydrolyse many oxides, halides, hydrides, carbides, nitrides, phosphides, carbonates etc. to give an acid or a base or both as shown below :
\[S{{O}_{2}}+{{H}_{2}}O\to {{H}_{2}}S{{O}_{3}}\]
\[M{{g}_{3}}{{N}_{2}}+6{{H}_{2}}O\to 3Mg{{(OH)}_{2}}+2N{{H}_{3}}\]
\[Ca{{H}_{2}}+2{{H}_{2}}O\to Ca{{(OH)}_{2}}+2{{H}_{2}}\]
\[CaO+{{H}_{2}}O\to Ca{{(OH)}_{2}}\]
\[N{{a}_{2}}C{{O}_{3}}+2{{H}_{2}}O\to 2NaOH+{{H}_{2}}C{{O}_{3}}\]
\[SiC{{l}_{4}}+4{{H}_{2}}O\to Si{{(OH)}_{4}}+4HCl\]
\[C{{a}_{3}}{{P}_{2}}+6{{H}_{2}}O\to 3Ca{{(OH)}_{2}}+2P{{H}_{3}}\]
\[Ca{{C}_{2}}+2{{H}_{2}}O\to Ca{{(OH)}_{2}}+{{C}_{2}}{{H}_{2}}\]
(v) Water forms hydrates with metal salts : There are three main types of hydrates.
(a) Compounds in which water molecule are co-ordinated to the metal ion (complex compounds) \[[Ni(O{{H}_{2}})]{{(N{{O}_{3}})}_{2}},\] \[Fe{{(O{{H}_{2}})}_{6}}]C{{l}_{3}}\] etc.
(b) Compound in which water molecule may be hydrogen bonded to oxygen to form oxo-anion. For example in \[CuS{{O}_{4}}.5{{H}_{2}}O\], 4 molecules of water are co-ordinated to \[C{{u}^{2+}}\] while the fifth molecule is hydrogen bonded to \[SO_{4}^{2-}\] ion.
(c) In some compounds, water molecule occupies, interstitial sites in the crystal lattice e.g., \[BaC{{l}_{2}}.2{{H}_{2}}O\].
(5) Hard and Soft water
Water which produces lather with soap solution readily is called soft water. e.g. distilled water, rain water and demineralised water.
Water which does not produce lather with soap solution readily is called hard water. e.g. sea water, river water, well water and tap water.
(i) Cause of hardness of water : The hardness of water is due to the presence of bicarbonates, chlorides and sulphates of calcium and magnesium.
Hard water does not produce lather because the cations \[(C{{a}^{+2}}\text{and}\,M{{g}^{+2}})\]present in hard water react with soap to form insoluble precipitates,
\[\underset{From\,hard\,water}{\mathop{{{M}^{+2}}}}\,+\underset{Sodium\,stearate(soap)}{\mathop{2{{C}_{17}}{{H}_{35}}COONa}}\,\to \underset{Metal\,stearate(PPt.)}{\mathop{{{({{C}_{17}}{{H}_{35}}COO)}_{2}}M}}\,+2N{{a}^{+}}\]
Where M = Ca or Mg
Therefore, no lather is produced until all the calcium and magnesium ions are precipitated. This also results into wastage of lot of soap.
(ii) Type of hardness of water : The hardness of water is of two types,
(a) Temporary hardness : This is due to the presence of bicarbonates of calcium and magnesium. It is also called carbonate hardness.
(b) Permanent hardness : This is due to the presence of chlorides and sulphates of calcium and magnesium. It is also called non-carbonate hardness.
(iii) Softening of water : The process of the removal of hardness from water is called softening of water.
(a) Removal of temporary hardness : It can be removed by the following methods,
\[\underset{Cal.\,bicarbonate}{\mathop{Ca{{(HC{{O}_{3}})}_{2}}}}\,\xrightarrow{Heat}\underset{PPt.}{\mathop{CaC{{O}_{3}}}}\,+C{{O}_{2}}+{{H}_{2}}O\]
\[\underset{Mag.\,bicarbonate}{\mathop{Mg{{(HC{{O}_{3}})}_{2}}}}\,\xrightarrow{Heat}\underset{PPt.}{\mathop{MgC{{O}_{3}}}}\,+C{{O}_{2}}+{{H}_{2}}O\]
\[\underset{\text{Soluble}}{\mathop{Ca{{(HC{{O}_{3}})}_{2}}}}\,+\underset{\text{Lime}}{\mathop{Ca{{(OH)}_{2}}}}\,\xrightarrow{{}}\underset{\text{Insoluble}}{\mathop{2CaC{{O}_{3}}}}\,\downarrow +2{{H}_{2}}O\]
\[\underset{\text{Soluble}}{\mathop{Mg{{(HC{{O}_{3}})}_{2}}}}\,+\underset{\text{Lime}}{\mathop{Ca(O{{H}_{2}})}}\,\xrightarrow{{}}\underset{(\text{Insoluble})}{\mathop{MgC{{O}_{3}}\downarrow +CaC{{O}_{3}}}}\,\downarrow +2{{H}_{2}}O\]
(b) Removal of permanent hardness : Permanent hardness can be removed by the following methods,
\[CaC{{l}_{2}}+N{{a}_{2}}C{{O}_{3}}\xrightarrow{{}}\underset{ppt.}{\mathop{CaC{{O}_{3}}}}\,+2NaCl\]
\[MgS{{O}_{4}}+N{{a}_{2}}C{{O}_{3}}\xrightarrow{{}}\underset{ppt.}{\mathop{MgC{{O}_{3}}}}\,+N{{a}_{2}}S{{O}_{4}}\]
The permutit as loosely packed in a big tank over a layer of coarse sand. Hard water is introduced into the tank from the top. Water reaches the bottom of the tank and then slowly rises through the permutit layer in the tank. The cations present in hard water are exchanged for sodium ions. Therefore this method is also called ion exchange method.
\[\underset{\begin{smallmatrix} \text{Sodium} \\ \text{zeolite }\end{smallmatrix}}{\mathop{N{{a}_{2}}Z}}\,+\underset{\begin{smallmatrix} \text{(From hard} \\ \text{water)}\end{smallmatrix}}{\mathop{C{{a}^{+2}}}}\,\xrightarrow{{}}\underset{\begin{smallmatrix} \text{Cal} \\ \text{zeolite}\end{smallmatrix}}{\mathop{CaZ}}\,+2N{{a}^{+}}\]
\[\underset{\begin{smallmatrix} \text{Sodium } \\ \text{zeolite}\end{smallmatrix}}{\mathop{N{{a}_{2}}Z}}\,+\underset{\begin{smallmatrix} \text{(From hard } \\ \text{water)}\end{smallmatrix}}{\mathop{M{{g}^{+2}}}}\,\xrightarrow{{}}\underset{\begin{smallmatrix} \text{Magnesium} \\ \text{zeolite}\end{smallmatrix}}{\mathop{MgZ}}\,+2N{{a}^{+}}\]
where
\[Z=A{{l}_{2}}S{{i}_{2}}{{O}_{8}}.\,\,\,x{{H}_{2}}O\]
You need to login to perform this action.
You will be redirected in
3 sec
