Cyanides and Isocyanides
Category : JEE Main & Advanced
Hydrogen cyanide is known to exist as a tautomeric mixture.
\[H-C\equiv N\rightleftharpoons H-N\ {{=}^{\to }}C\]
Hence, it forms two types of alkyl derivatives which are known as alkyl cyanides and alkyl isocyanides.
\[\underset{\text{Alkyl Cyanide}}{\mathop{RC\equiv N}}\,\] \[\underset{\text{Alkyl isocyanide}}{\mathop{RN\ \ {{=}^{\to }}C}}\,\]
(1) Alkyl Cyanides
(i) Methods of preparation
(a) From alkyl halides : The disadvantage of this method is that a mixture of nitrile and isonitrile is formed.
\[\underset{\begin{smallmatrix} \text{Alkyl} \\ \text{halide} \end{smallmatrix}}{\mathop{RX}}\,+KCN(orNaCN)\to \underset{\begin{smallmatrix} \,\,\,\,\,\,\text{Nitrile} \\ \text{(Major product)} \end{smallmatrix}}{\mathop{RCN}}\,+\underset{\begin{smallmatrix} \,\,\,\,\,\,\text{Isonitrile} \\ \text{(Minor product)} \end{smallmatrix}}{\mathop{RNC}}\,\]
(b) From acid amides : \[RCON{{H}_{2}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{P}_{2}}{{O}_{5}}}}}\,RCN\]
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\xrightarrow{{{P}_{2}}{{O}_{5}}}\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}CN}}\,+{{H}_{2}}O\]
Industrially, alkyl cyanides are prepared by passing a mixture of carboxylic acid and ammonia over alumina at \[500{}^\circ C\].
\[\underset{\text{Acid}}{\mathop{RCOOH}}\,+N{{H}_{3}}\to \underset{\text{Ammonium salt}}{\mathop{RCOON{{H}_{4}}}}\,\underset{{{H}_{2}}O}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,\underset{\text{Amide}}{\mathop{RCON{{H}_{2}}}}\,\underset{{{H}_{2}}O}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,\underset{\text{Alkyl cyanide}}{\mathop{RCN}}\,\]\[\underset{\text{Amide}}{\mathop{RCON{{H}_{2}}}}\,\underset{{{H}_{2}}O}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,\underset{\text{Alkyl cyanide}}{\mathop{RCN}}\,\]
(c) From Grignard reagent
![]()
![]()
(d) From primary amines : Primary amines are dehydrogenated at high temperature to form alkyl cyanides. This is also a commercial method.
\[\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\underset{500{}^\circ C}{\mathop{\xrightarrow{Cu\,or\,Ni}}}\,RCN+2{{H}_{2}}\]
\[\underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,\underset{500{}^\circ C}{\mathop{\xrightarrow{Cu\,or\,Ni}}}\,\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}CN}}\,+2{{H}_{2}}\]
(e) From oximes :
\[\underset{\text{Aldoxime}}{\mathop{R-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=NOH}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{P}_{2}}{{O}_{5}}}}}\,\underset{\text{Alkyl cyanide}}{\mathop{R-CN}}\,+{{H}_{2}}O\]
(ii) Physical properties
(a) Alkyl cyanides are neutral substance with pleasant odour, similar to bitter almonds.
(b) Lower members containing upto 15 carbon atoms are liquids, while higher members are solids.
(c) They are soluble in water. The solubility decreases with the increase in number of carbon atoms in the molecule.
(d) They are soluble in organic solvents.
(e) They are poisonous but less poisonous than HCN
(iii) Chemical properties
(a) Hydrolysis
\[\underset{\begin{smallmatrix} \,\,\text{Alkyl} \\ \text{cyanide} \end{smallmatrix}}{\mathop{RCN}}\,\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Amide}}{\mathop{RCON{{H}_{2}}}}\,\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Acid}}{\mathop{RCOOH}}\,+N{{H}_{3}}\]
\[\underset{\begin{smallmatrix} \text{Methyl} \\ \text{cyanide} \end{smallmatrix}}{\mathop{C{{H}_{3}}CN}}\,\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\]\[\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+N{{H}_{3}}\]
(b) Reduction : When reduced with hydrogen in presence of Pt or Ni, or \[LiAl{{H}_{4}}\] (Lithium aluminium hydride) or sodium and alcohol, alkyl cyanides yield primary amines.
\[\underset{\text{Alkyl cyanide}}{\mathop{RCN}}\,\xrightarrow{4H}\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
However, when a solution of alkyl cyanides in ether is reduced with stannous chloride and hydrochloric acid and then steam distilled, an aldehyde is formed (Stephen's reaction).
\[R-C\equiv N\underset{[2H]}{\mathop{\xrightarrow{{SnC{{l}_{2}}}/{HCl}\;}}}\,\underset{\text{Imine hydrochloride}}{\mathop{RCH=NH.HCl}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Aldehyde}}{\mathop{RCHO}}\,\ +N{{H}_{4}}Cl\]
(c) Reaction with Grignard reagent : With grignard's reagent, an alkyl cyanide forms a ketone which further reacts to form a tertiary alcohol.
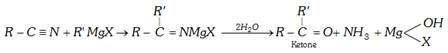
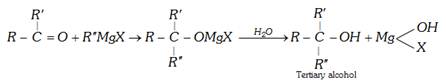
(d) Alcohololysis :
\[\underset{\begin{smallmatrix} \,\text{Alkyl} \\ \text{cyanide} \end{smallmatrix}}{\mathop{RCN}}\,+\underset{\text{Alcohol}}{\mathop{{R}'OH}}\,+HCl\to \underset{\text{imido ester}}{\mathop{\left[ \overset{\,\,\,\overset{+}{\mathop{N}}\,{{H}_{2}}}{\mathop{R-\overset{|\,|}{\mathop{C}}\,-O{R}'}}\, \right]}}\,\,\,C{{l}^{-}}\]\[\xrightarrow{{{H}_{2}}O}\underset{\text{Ester}}{\mathop{RCOO{R}'}}\,\ +N{{H}_{4}}Cl\]
(iv) Uses : Alkyl cyanides are important intermediates in the organic synthesis of a large number of compounds like acids, amides, esters, amines etc.
(2) Alkyl Isocyanides
(i) Methods of preparation
(a) From alkyl halides :
\[\underset{\text{Alkyl halide}}{\mathop{R-X}}\,+AgCN\to \underset{\begin{smallmatrix} \,\,\text{Isocyanide} \\ \,\,\text{(Isonitrile)} \\ \text{Main product} \end{smallmatrix}}{\mathop{RNC}}\,+\underset{\begin{smallmatrix} \,\,\,\,\text{Cyanide} \\ \,\,\,\,\text{(Nitrile)} \\ \text{Minor pro}\text{duct} \end{smallmatrix}}{\mathop{RCN}}\,\] \[\underset{\text{Methyl chloride}}{\mathop{C{{H}_{3}}Cl}}\,+AgCN\to \underset{\begin{smallmatrix} \text{Methyl isocyanide} \\ \,\,\text{(Main product)} \end{smallmatrix}}{\mathop{C{{H}_{3}}NC}}\,+C{{H}_{3}}CN\]
(b) From primary amines (Carbylamine reaction) :
\[\underset{\text{Primary amine}}{\mathop{RN{{H}_{2}}}}\,+\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,+3KOH\to \underset{\text{Isocyanide}}{\mathop{RNC}}\,+3KCl+3{{H}_{2}}O\]
(c) From N-alkyl formamides :
\[\underset{N-\text{alkyl formamide}}{\mathop{R-NH-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-H}}\,\underset{\text{Pyridine}}{\mathop{\xrightarrow{POC{{l}_{3}}}}}\,\underset{\text{Isocyanide}}{\mathop{R-N\ \ {{=}^{\to }}C}}\,+{{H}_{2}}O\]
(ii) Physical properties
(a) Alkyl isocyanides are colourless, unpleasant smelling liquids.
(b) They are insoluble in water but freely soluble in organic solvents.
(c) Isonitriles are much more poisonous than isomeric cyanides.
(iii) Chemical properties
(a) Hydrolysis :
\[\underset{\text{Alkyl isocyanide }}{\mathop{RN{{=}^{\to }}C+}}\,2{{H}_{2}}O\xrightarrow{{{H}^{+}}}\underset{\text{Primary amine}}{\mathop{RN{{H}_{2}}+}}\,\underset{\text{Formic acid}}{\mathop{HCOOH}}\,\]
(b) Reduction : \[\underset{\text{Alkyl isocyanide}}{\mathop{R-N{{=}^{\to }}C+}}\,4H\underset{{{300}^{o}}C}{\mathop{\xrightarrow{Ni}}}\,\underset{\text{secondary amine}}{\mathop{RNHC{{H}_{3}}}}\,\]
(c) Action of heat : When heated for sometime at \[{{250}^{o}}C\], a small amount of isonitrile changes into isomeric nitrile.
\[RNC\xrightarrow{\text{heat}}RCN\]
(d) Addition reaction : Alkyl isocyanide give addition reactions due to presence of unshared electron pair on carbon atom.
\[R:N:::C:\] or \[R-\overset{+}{\mathop{N}}\,\equiv \overset{-}{\mathop{C}}\,\]
The following are some of the addition reactions shown by alkyl isocyanides.
\[RNC\ \ +\underset{\text{(Halogen)}}{\mathop{{{X}_{2}}}}\,\to \underset{\begin{smallmatrix} \text{Alkyl iminocarbonyl } \\ \text{ halide} \end{smallmatrix}}{\mathop{RNC{{X}_{2}}}}\,\]
\[RNC+S\to \underset{\begin{smallmatrix} \text{ Alkyl } \\ \text{isothiocyanate} \end{smallmatrix}}{\mathop{RNCS}}\,\]; \[RNC+HgO\to \underset{\begin{smallmatrix} \,\,\,\text{Alkyl} \\ \text{isocyanate} \end{smallmatrix}}{\mathop{RNCO}}\,+Hg\]
(iv) Uses : Due to their unpleasant smell, alkyl isocyanides are used in detection of very minute leakage. Carbylamine reaction is used as a test for the detection of primary amino group.
Comparison of Alkyl Cyanides and Alkyl Isocyanides
| Test | Ethyl cyanide | Ethyl isocyanide |
| Smell | Strong but pleasant | Extremely unpleasant |
| Dipole moment | More \[(\approx \,4D)\] | Less \[(\approx \,3D)\] |
| B.P. | \[{{98}^{o}}C\] (i.e. High) | \[{{78}^{o}}C\] (i.e. low) |
| Solubility in water. | Soluble | Insoluble |
| Hydrolysis with acids | Gives propionic acid (Acid, in general) | Give ethyl amine (\[{{1}^{o}}\] amine, in general) |
| Hydrolysis with alkalies | Same as above | No action |
| Reduction | Gives propylamine (\[{{1}^{o}}\] amine, in general) | Gives ethylmethyl amine (\[{{2}^{o}}\] amine, in general) |
| Stephen's reaction | Gives propionaldehyde (Aldehyde, in general) | Does not occur |
| Heating \[(250{}^\circ C)\] | No effect | Ethyl cyanide is formed |
You need to login to perform this action.
You will be redirected in
3 sec
